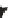Geoscience Reference
In-Depth Information
Composition of
t
he first liquids
Forsterite
+ liquid
Quartz
+
liquid
En. +
liq.
Forsterite
+ enstatite
Quartz +
enstatite
Forsterite
Enstatite
Quartz
Figure 11.3
The effect of pressure on the composition of the first melt (bold line). With depth, peritectic
reactional melting, producing basalts with a fairly high silica content (tholeiites), gives way to
drifts toward that of the initial solid. In the mantle, an increase in temperature and
degree of melting produces Mg-rich rocks. Komatiites are high-magnesian lavas found
only in Archean terranes (except for the small Tertiary flows on Gorgona Island). They
are explained by very intense melting of the mantle of the order of 50% and are thus
evidence of the very high temperature of some parts of the mantle during the earliest
ages of the Earth.
4.
Water and carbon dioxide content of the source
. The effect of water is similar to that
of elements with low melting points (e.g. alkali elements). Water may form hydrous
minerals such as mica and amphibole in the upper mantle and a number of unnamed
minerals (“alphabet” phases known by letters) at depth, but it is also dissolved in the
lattice of the so-called “nominally anhydrous minerals” such as olivine and pyroxene
and greatly reduces their viscosity. Water is also very soluble in melts because it breaks
the Si-O-Si double bond into two Si-OH single bonds. The presence of such “im-
purities” lowers the melting point of the rocks (
Appendix C
,
(C.27
)
) in proportion of
their molar fraction: since water has quite a small molar weight (18), its effect per
weight percent on the melting point is much larger that that of other minor incom-
patible elements such as Ti or P. As long as a gas phase is absent, water behavior is
otherwise similar to that of an ordinary incompatible element. It is noteworthy that the
H
2
O/Ce ratio is practically constant at 300 in basaltic magmas from different environ-
ments (mid-ocean ridges, oceanic islands, subduction zones) when measurements are
made on melt inclusions within crystals, i.e. from non-degassed samples. As water is
nonetheless the most abundant impurity, it soon reaches saturation and forms a vapor
phase as the magma rises and separates out from the melt. From this point, if the vapor
phase is dominated by water, the mechanical equilibrium of vapor and the surrounding
rock requires the water pressure to be equal to the pressure of the surrounding rock and