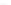Geoscience Reference
In-Depth Information
−
15 (rain)
0
0
+8
+8
+4
−
5
+4
350
°
C
700
C
°
Granitic pluton
Figure 10.5
Geothermal system controlled by the emplacement of a granite pluton: rain water infiltrates and
when heated in proximity to the intrusion becomes less dense and percolates back to the surface.
Values shown (in
‰
) are those of
18
O of rock (and of precipitation). Rain water is strongly
depleted in
18
O and changes the values of rocks, initially here at
δ
18
O,
depending on temperature and the water/rock ratio. Crystallization of the granite magmas may
release magmatic waters.
8
‰
,tolowervaluesof
+
δ
From these solubility relationships, we gain some understanding of the mineral
associations present in the fractures observed in granitic and metamorphic base-
ments. Pegmatites, characterized by large crystals of albite and K-feldspars, precipitate
from relatively high-temperature hydrous fluids (400-600
◦
C), while the quartz veins,
which are commonly associated with ore deposits, form at lower temperatures (200
-400
◦
C).
Water-rock interactions may also leave a profound mark on the isotopic compositions
of the geothermal systems associated with magmatic provinces, such as Wairakei in New
Zealand, Larderello in Italy, and the Geysers of California. Felsic intrusions emplaced
at a few kilometers below the surface act as deep heat engines; they promote convec-
tion of the pore fluids and isotopic exchange between meteoric water and the rock matrix
hydrogen, the
18
O and
δ
δ
D of the magmatic intrusions themselves and of their coun-
try rocks are significantly shifted towards lower values upon exchange with meteoric
water.
The markedly marine signature of isotopic compositions of oxygen and hydrogen in
solutions from black smokers indicates that these formed by seawater infiltrating the edges
of the ridges and by reaction at depth with the still hot basalt. Hydrothermal reactions at
the mid-ocean ridges play an important role in the magnesium cycle and in controlling the
alkalinity of the ocean. It has been observed that water from the black smokers of the ocean
ridges is particularly acidic, i.e. its pH is much lower (typically 3) than that of deep ocean