Geoscience Reference
In-Depth Information
800
700
600
500
400
300
200
100
0
0
50
100
150
200
250
300
350
Water temperature (
°
C)
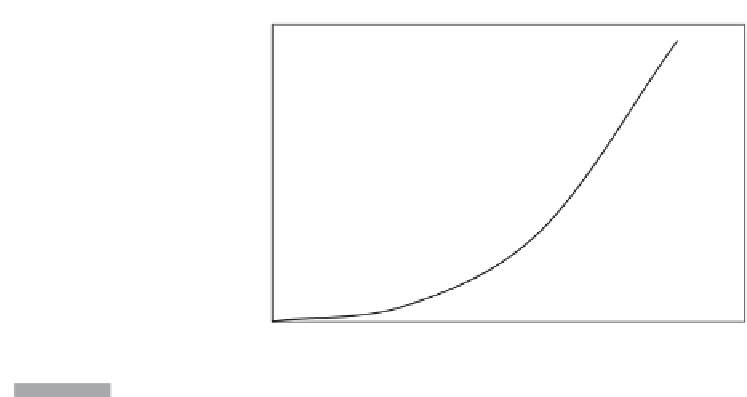
Figure 10.4
Solubility of silica in water. Silica contents of thermal waters can be used as a thermometer to a
temperature of about 220
◦
C, and thus indicate the depth of equilibration. Above this
temperature, amorphous silica precipitates as the fluid rises.
commonplace to utilize analysis of the water-rock equilibria for hydrothermal reaction
thermometry, particularly K/Na fractionation induced by the exchange reaction between
feldspar and the solution:
K
+
Na
+
K
+
Na
+
+
⇔
+
(10.10)
(solution)
(feldspar)
(feldspar)
(solution)
Assuming that feldspars in the reservoir zone of the solution have the compositions of
common feldspars from granites and metamorphic rocks, the mass action law yields the
equation:
Na
+
K
+
solution
=
908
T
−
log
10
0.70
(10.11)
The sodium and potassium contents of a hydrothermal solution therefore allow an equilib-
rium temperature with feldspar to be deduced. Solutions can also dissolve minerals without
any reaction product: this is the case for silica, the content of which in spa water is used as
a thermometer by writing the thermodynamic law of saturation (
Appendix C
)
:
dln[SiO
2
]
solution
d
=
H
R
(10.12)
(
1
/
T
)
where
H
is the heat of dissolution of silica in water. By means of a few approximations
and by introducing experimental values, the silica thermometer equation is:
1306
T
log
10
[SiO
2
]
solution
=−
+
0.38
(10.13)
(note the logarithms with different bases). Assuming that there is surplus silica, which is
true for most continental rocks, the measurement of the silica content of thermal water
gives, with this equation, the equilibration temperature of the solutions with the deep rocks