Geoscience Reference
In-Depth Information
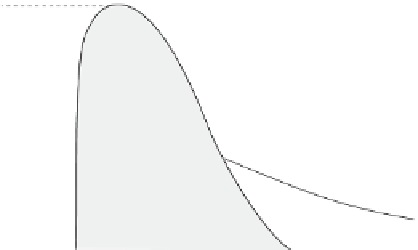
Critical point
NaCl brine
One-phase fluid
T
2
>
T
cr
T
cr
Critical point
pure water
P
cr
T
1
<
T
cr
Liquid
Vapor
Two-phase fluid
Molar volume
100
◦
C, boiling of pure water upon
decompression proceeds by phase separation (the dotted line shows a metastable trajectory). The
horizontal segment connects the two points representing the molar volumes of the liquid and the
vapor. At the critical temperature
T
cr
, these molar volumes become equal and transition from
liquid to vapor takes place in a continuous manner. The state referred to as one-phase fluid is a
continuum between the liquid state and the gas state. The lowest temperature and pressure
T
cr
and
P
cr
at which this can happen define the critical point. Note that adding salt to water
significantly increases both
T
cr
and
P
cr
.
Figure 10.3
The critical behavior of aqueous solutions. At
T
1
=
the continents give an imperfect sampling of hydrothermal solutions: because the boil-
ing point of hydrous solutions increases from 100
◦
C at ambient pressure to more than
350
◦
C at a few kilometers below the surface, the compositions and temperatures of ther-
mal springs do not faithfully represent the properties of the solutions in contact with deep
rock. Most hydrothermal springs therefore have been affected by intense boiling, to which
they are subjected as they rise to the surface. In contrast, the emergence temperatures
of the solutions spouted by the black smokers along mid-ocean ridges can reach 400
◦
C
because of the high pressure prevailing at the ocean floor (200-450 atmospheres), and bet-
ter represent the original hydrothermal solutions. Many geologically (and economically)
important hydrothermal reactions take place at even higher temperatures (400-600
◦
C).
High-temperature solutions are often trapped in some minerals, such as quartz, in the form
of fluid inclusions.
In order to understand hydrothermal reactions, we must examine phase changes and
critical phenomena in high-temperature hydrous solutions. Let us first take some liquid
water at 100
◦
C in an autoclave pressurized by a neutral gas such as air and progressively
changed into vapor. For the time it takes to evaporate the liquid, we have a two-phase fluid
with liquid and vapor separated by a visible interface. If we measure the molar volume, it
slightly increases upon decompression in the liquid domain, remains fixed during boiling,