Geoscience Reference
In-Depth Information
Naturally abundant nuclide
(stable or long lived)
Naturally rare or artificial nuclide
(short lived)
Neutron-poor nuclid
es
Z
=
N
Neutron-rich nuclides
Neutron number,
N
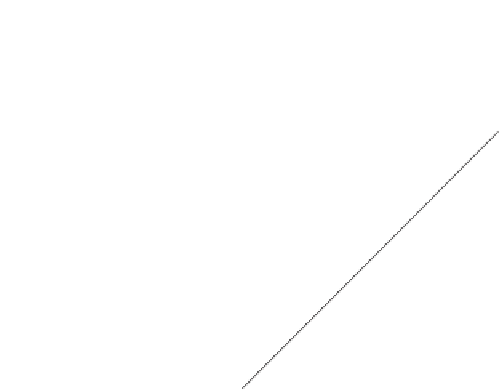
Figure 1.1
Chart of the nuclides (overview). The light stable elements have approximately the same number
Z
of protons and number
N
of neutrons but the heavier stable elements deviate towards the
neutron-rich side according to
(1.2)
:
this relationship defines the valley of stability. Elements that
depart significantly from this rule are unstable (radioactive).
and this describes the so-called “liquid drop” model of the nucleus. The constants
a
,
b
,
c
,
and
d
can be adjusted to fit laboratory data. As a first approximation, the volume of the
nucleus is proportional to
A
, its radius to
A
1
/
3
, and its surface area to
A
2
/
3
. The first
term on the right-hand side expresses the volume energy, which is proportional to the
number of nucleons; the second term is the surface energy, which subtracts the uncom-
pensated attraction of the nucleons located near the surface of the nucleus; the third term
expresses that, for a given
A
, the nuclear attraction between proton and neutron is slightly
stronger than proton-proton and neutron-neutron attraction; the fourth term accounts for
electrostatic energy which is inversely proportional to the distance between the neighbor-
ing charges of the protons. The locus of minimum energy, in the
N
,
Z
plot of
Fig. 1.1
,
which is known as the “valley of stability,” is obtained by minimizing
(1.1)
with respect to
Z
, and is conveniently represented by the equation:
2
A
Z
=
(1.2)
0.031
A
2
/
3
4
+
<
≈
/
For light elements (
Z
40), the term in
A
at the denominator is very small, so
Z
A
2
≈
and therefore
N
Z
. At higher masses, electrostatic repulsion between protons gets
Z
. One easily finds that for
238
U,
Z
= 92 which is correct.
Nuclei with
N
and
Z
too far from this valley of stability are unstable and are said to be
radioactive. An isotope is radioactive if its nucleus undergoes spontaneous change such as
occurs, for instance, when alpha particles (two protons and two neutrons) or electrons are
emitted. It changes into a different isotope, referred to as radiogenic, by giving out energy,
usually in the form of gamma radiation, some of which is harmful for humans. Several
internet sites provide tables of all stable and radioactive nuclides. The vast majority of
stronger and
N
>