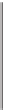Geoscience Reference
In-Depth Information
+25
pe (pH
=
7)
O
2
+20
H
2
O
Oxidation
+15
NO
-
MnO
2
+10
Mn
2+
Reduction
+5
N
2
Fe(OH)
3
Fe
2+
0
2-
SO
4
HS
-
HCO
-
CH
4
-5
HCO
-
+
NH
4
CH
2
O
-10
Food
Reductant (electron donor)
Figure 8.8
Dominance plot of the major species present in sediments and pore waters and which can act as
either electron donor (reductant) or acceptor (oxidant). Low-pe organic matter CH
2
O (food) is
supposed to be present in excess. The energy driving its oxidation (upward) into HCO
3
,is
obtained from the dissimilatory reduction (downward) of the oxidants shown at the high-pe end
of each bar.
with pore water where, upon re-oxidation, it contributes to the small diagenetic compo-
nent of polymetallic nodules. In contrast, ferrous iron reacts with the sulfur liberated by
the dissimilatory reduction of sulfate and precipitates as pyrite FeS
2
, a particularly com-
mon diagenetic mineral in sedimentary rocks with a high organic matter content. At even
greater depth and if temperature is adequate, leftover organic material may be reduced to
methane. All these reactions are biologically mediated. Overall, pore waters seeping out
from sediments rich in organic matter and nitrogen are not only highly reduced but also rich
in metals. Because porous flow is largely controlled by the rate of burial, this is certainly
an important control factor of these processes.