Geoscience Reference
In-Depth Information
0.0031
0.0029
0.0027
100
200
0.0025
500
0.0023
0.0021
0.0019
0.0019
0.0020
0.0021
0.0022
0.0023
0.0024
0.0025
0.0026
Σ
CO
2
(mol
kg
−
1
)
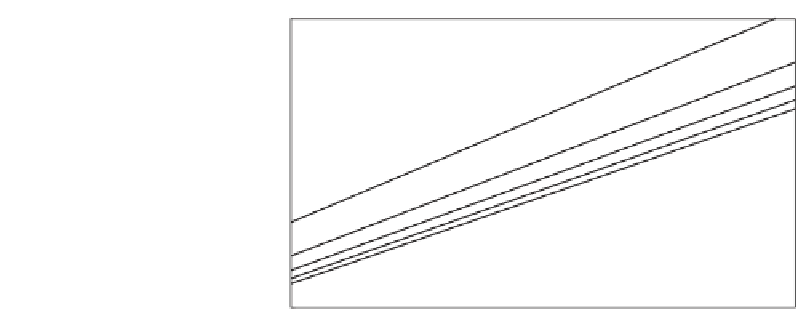
Figure 7.7
The relationship between alkalinity and the sum of carbonates in the ocean for different values
of CO
2
content in the atmosphere, expressed in parts per million volume on the straight lines
about 350 ppmv CO
2
. Ocean and atmosphere are close to equilibrium.
be calculated either in surface water at a known pressure
P
CO
2
, or in deep water for a fixed
value of
CO
2
, by introducing the values of
HCO
3
and
CO
2
3
obtained through
(7.35)
Let us consider a different example relevant to our oceanic system in which the calcium
concentration of the ocean is fixed by carbonate saturation and seawater maintained at a
two equations expressing saturation in calcite:
Ca
2
+
CO
2
3
=
K
s
(7.37)
and CO
2
solubility:
[H
2
CO
3
]
=
k
CO
2
P
CO
2
(7.38)
The total concentration
CO
2
of carbonate ions becomes:
1
H
+
K
2
H
+
2
K
1
K
2
K
s
Ca
2
+
CO
2
=
+
+
(7.39)
while
P
CO
2
is given by:
H
+
2
Ca
2
+
K
s
K
1
K
2
k
CO
2
P
CO
2
=
(7.40)
The carbonate system therefore has two degrees of freedom which represent different geo-
logical conditions. Controls by suitable pairs of parameters, such as constant alkalinity and
CO
2
(deep ocean) or calcite saturation and
P
CO
2
(surface ocean) represent typical cases.
Two of the parameters are imposed and all of the other variables follow from there.
Reconstructing the composition of the ancient oceans therefore comes down to finding
geochemical tracers (proxies) for evaluating one or another of these parameters. Much hope