Geoscience Reference
In-Depth Information
Decreasing temperature
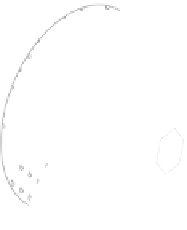
Figure 5.6
Evolution of chemical exchanges between the minerals co-existing in a rock upon cooling. A fluid
interstitial phase is assumed to be present. At high temperatures (dark tones), all minerals
actively exchange material. When temperature drops below the closure temperature of one
mineral phase, exchanges between this mineral and its environment comes to a halt (light
tones). Note the strong differences between this representation of mineral compositions and that
entailed by equilibrium between the co-existing mineral phases.
of the rock and its interstitial fluid comes to an end. The energy is no longer at its lowest
point but, because diffusion is thermally activated, the cost of reaching the true minimum
is higher than the energy available in the system: the system is caught in a sequence of
local minima, a series of states called metastable. Each mineral in turn withdraws from the
energy fair until the last mineral itself stops equilibrating with the fluid. This is Nature's
mineral performance of Haydn's Farewell Symphony in which each artist, at the conclu-
sion of his part, blows the candlelight set on his podium. A rock can rarely be regarded
as a true mineral assemblage unless all the mineral phases reach their closure temperature
in a very short time interval. This perception is of course true for the exchange of both
elements and isotopes. Thermometers and barometers follow the same rules as chronome-
ters: they record the temperature and the pressure at the time of the last equilibration
between the phases involved in the exchange. A temperature of 700
◦
C obtained between a
garnet-clinopyroxene pair in an eclogite only measures the lowest temperature prevail-
ing when these two minerals last exchanged. More informative observations are those
derived by
in situ
analysis, when the adjacent rims of both minerals are measured. Still,
uncertainties remain about what the numbers actually mean. The same holds true for oxy-
gen isotope thermometry which, for these reasons, fell into near oblivion more than three
decades ago.
Minerals with very low diffusivity are therefore in very high demand because their clo-
sure temperature is high. We have seen that zircon is an excellent chronometer for the
U-Pb systems, which it owes to a closure temperature for Pb diffusion in excess of 900
◦
C
(Cherniak
et al.
,
2001
). Zircon crystallizes early in granites. It nevertheless preserves the
oxygen isotope composition of its parent magma throughout the severe disturbances
imposed by the intense percolation of fluids at temperatures of 400-600
◦
C recorded in
major minerals such as quartz, feldspars and micas. This property has found an impor-
tant application in demonstrating that the source of early Archean granites, in which the