Biology Reference
In-Depth Information
19.2.5.2
High-performance liquid chromatography analysis
The kinetics of the reaction of compounds with cross-linked stabilized MTs and with
unassembled tubulin can be measured by HPLC.
1.
Prepare 20 mg tubulin in PEDTA buffer as described in
Section 1.1
. Add 1.5 mM
MgCl
2
to the buffer and increase the concentration of GTP to 1 mM.
2.
Incubate 20
m
M compounds for different times with either 25
m
M tubulin in
M cross-linked MTs in GAB buffer.
3.
After the desired incubation, centrifuge the samples (37
C, 20 min), separate the
pellet, and resuspend the supernatant in 10 mM NaPi buffer.
4.
Add an internal standard and extract compounds from the supernatant three times
with one reaction volume of dichloromethane and vacuum dry.
5.
Resuspend the sample in solvent and analyze by HPLC as described in
Section 2.3
, steps 5 and 6, and
Section 2.3.1
.
PEDTA buffer or 25
m
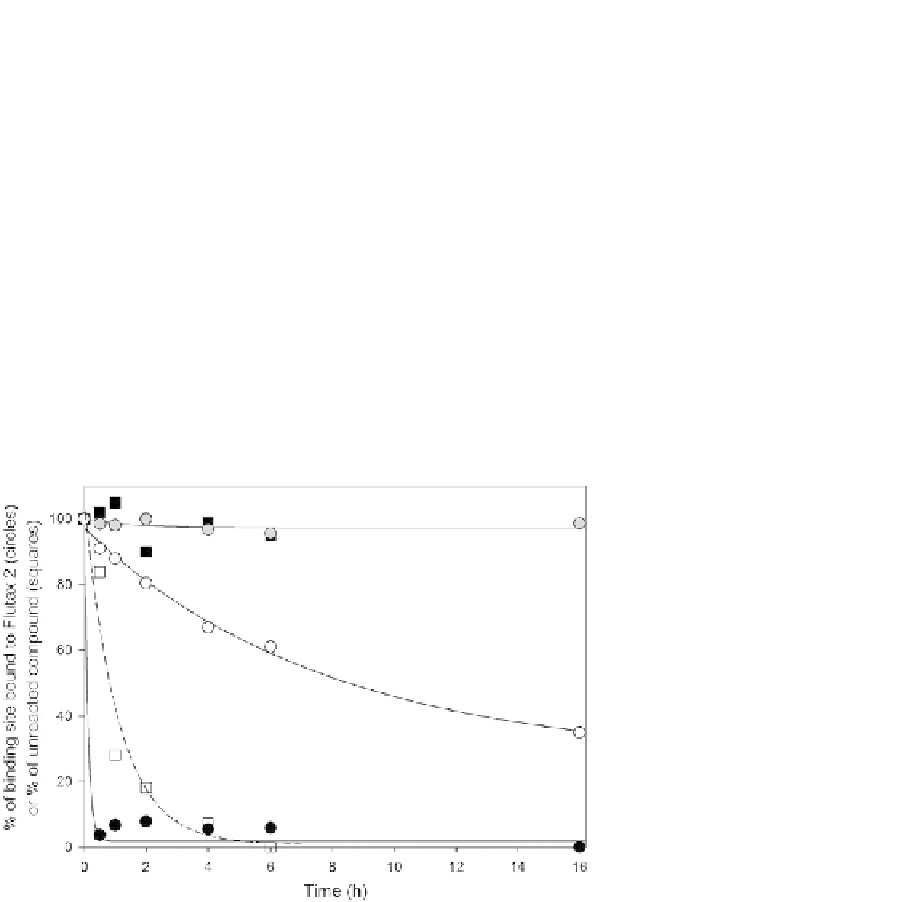
FIGURE 19.4
Binding kinetics of ZMP and dactylolide to the microtubule. The percentage of binding sites
bound by Flutax-2 (circles) and the percentage of unreacted compound detected by HPLC
(squares): ZMP (black circles), dactylolide (white circles), DMSO control (gray circles),
dactylolide (white squares), and dactylolide control with no MTs (black squares). Solid
lines represent the loss of available binding sites, and the dashed line represents the fit for
decay of unbound dactylolide. The difference in the two measurements for dactylolide
indicates that the covalent reaction is slow. All FTX-2 was displaced by ZMP, and all ZMP was
found bound to the pellet in 30 min, indicating covalent binding immediately follows the
noncovalent reaction.
Graph modified from
Field et al. (2012)
with permission from the publisher.