Biology Reference
In-Depth Information
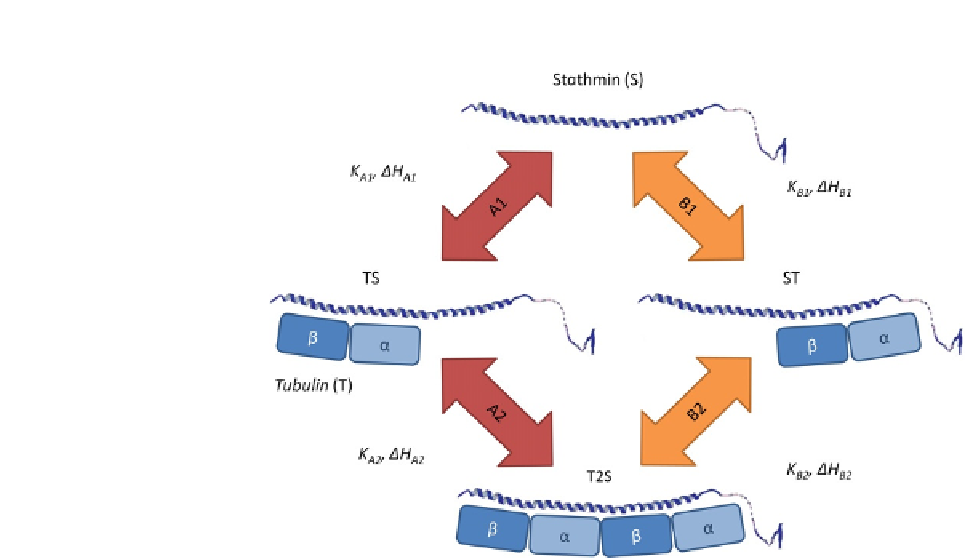
FIGURE 18.6
Schema of tubulin binding to stathmin. The general model of tubulin interaction with stathmin
supposes or assumes the existence of two nonequal interacting sites described by six
independent parameters
K
A1
,
K
B1
,
K
A2
,
DH
A1
,
DH
B1
, and
DH
A2
(although in the full
thermodynamic cycle
K
B2
,
DH
B2
are not independent).
enthalpy of binding for the second site (
H
B
2
). In this case,
treating a binding isotherm with a model of one-set-of-sites results in the determi-
nation of wrong binding constants (
K
0
) for each site. However, in both cases, the
overall reaction can be written as follows:
D
H
A
1
¼D
H
A
2
¼D
H
B
1
¼D
2T
þ
S
T2S
K
T2S
; D
ð
H
T2S
Þ
(18.3)
>
with an overall constant of formation of the T2S complex
K
T2S
¼
K
0
*
K
0
¼
K
A
1
*
K
A
2
¼
K
B
1
*
K
B
2
.
Considering all the earlier evidence about the cooperativity, the current structural
view of the asymmetric T2S complex (
Gigant et al., 2000
), and the fact that ITC cannot
distinguish between two binding sites that would have the same
H
of binding of individual tubulin to stathmin changes neither with the position on
stathmin, nor with the presence of another tubulin on stathmin. A possible way to ex-
plain how
D
H
,itislikelythat
D
H
can be similar despite the asymmetry of stathmin would be to consider
that the heat exchanged during tubulin-stathmin interaction is mostly due to the lateral
interaction of tubulin with the long alpha helix of stathmin, with very little (negligible)
heat exchanged at the interface between
D
b
-tubulin and the consecutive
a
-tubulin or