Biology Reference
In-Depth Information
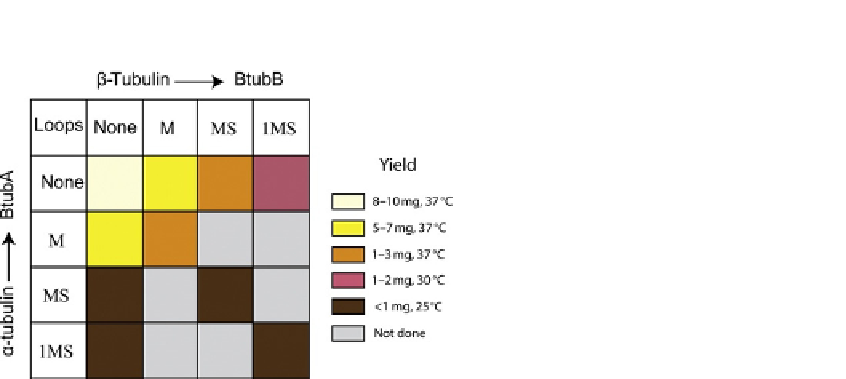
FIGURE 17.3
Recovery of BtubA/B chimera containing eukaryotic tubulin loop sequences 1, M, and S
in one or both subunits, following expression and purification as for wild-type BtubA/B
(
Martin-Galiano et al., 2011
).
This research was originally published in the Journal of Biological Chemistry. (2011) 286, 19789-19803
#
the
American Society for Biochemistry and Molecular Biology.
correct protein behavior, it is important to examine the BtubA/B polymer reversibil-
ity, monitoring disassembly by GTP (0.1 mM) exhaustion or upon excess GDP ad-
dition. Notice that this buffer does not support eukaryotic tubulin assembly, but we
have observed that both proteins polymerize with GTP in 20 mM Tris, 300 mM po-
tassium glutamate, 5 mM MgCl
2
, 1 mM EGTA, pH 7.5, at 30
C. Our results also
showed that BtubA/B polymerization is more weakly dependent on pH, temperature,
and divalent cations than eukaryotic tubulin (
Martin-Galiano et al., 2011
). This
method requires a relatively high amount of protein but directly monitors the fila-
ments assembly and disassembly dynamicity.
Sedimentation experiments allowmeasuring the amount of polymer formation by
centrifuging assembled reaction mixtures and by carefully quantifying pellets and
supernatants in stained SDS-PAGE gels. We usually prepare 0.1 mL samples and
after an incubation period, centrifuge them at 250,000
g
10 min in a Beckman
Optima table-top ultracentrifuge. The advantage of this method is that it requires less
amount of protein and makes monitoring a wide range of conditions in a single
experiment possible.
Figure 17.4
shows an example of BtubA/B polymerization
analyzed by sedimentation (top) and the comparison using both sedimentation
and the maximal light scattering signal for critical concentration measurement
(Cr
M).
Notice the importance of confirming the morphology of the BtubA/B polymers
by negative stain electron microscopy of unfixed samples. This is necessary to truly
relate sedimentation and light-scattering profiles to the formation of ordered
¼
0.9
m