Biology Reference
In-Depth Information
nucleotide contribution (0.68
0.08 GTP and 1.01
0.11 GDP bound per
BtubA/B;
Martin-Galiano et al., 2011
).
BtubA/B prepared in this manner contains BtubA and BtubB in equimolar ratio and
is
100% active in polymerization above a critical concentration (Cr). Wild-type
BtubA/B can also be purified for routine purposes performing the polymerization
cycle on the high-speed supernatant of the cell lysate (following step 1) instead of
doing it on the purified protein (following step 3). These preparations gave a some-
what higher yield of a similar BtubA/B,
90% active in polymerization above Cr.
However, we found that this procedure is not suited for BtubA/B constructs (below)
with modified polymerization activity due to mutations or insertions.
17.2.2
BtubA and BtubB purification
Because
btubA
and
btubB
genes are cloned for bicistronic expression, both proteins
are purified together. Nevertheless, for some experiments, it is necessary to use iso-
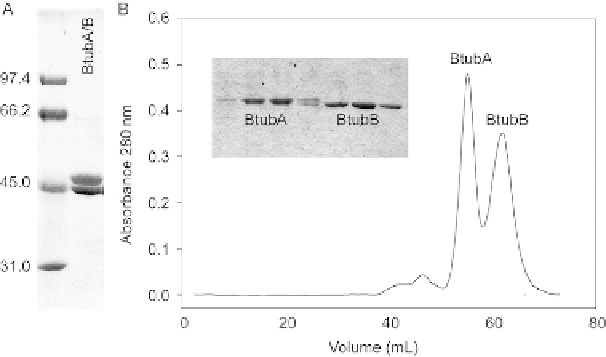
lated BtubA and BtubB. For this purpose, we perform the size exclusion chromatog-
raphy (step A3 above, using nonpolymerized BtubA/B) with a prepacked Sephacryl
S200 HR 26/60 HP column (GE Healthcare) at 4
C (run at 0.5 mL/min, taking 2 mL
fractions). The BtubA and BtubB subunits separate despite their similar size, possi-
bly because of BtubA dimerization or BtubB interaction with the gel matrix
(
Fig. 17.2
). The nucleotide content measurements of BtubA and BtubB samples an-
alyzed by HPLC after protein precipitation with ice-cold 0.5N HCLO
4
showed that
FIGURE 17.2
(A) Purified bacterial tubulin BtubA/B in gel electrophoresis. Molecular weight markers (kDa)
are on the left lane. (B) Separation of BtubA and BtubB by gel chromatography in a Sephacryl
S200 column (Methods, B).