Biology Reference
In-Depth Information
The dissected nerve is transferred to a 100-mm Petri dish containing cold filtered
seawater (use 0.45-
m pore filters and maintain at 0-4
C) for fine dissection. We
prefer natural seawater to reveal axon damage that may affect transport, but some use
Ca
2
þ
-free artificial seawater for the fine dissection. The nerve bundle is fixed place
by wrapping the thread around 18-g hypodermic needles inserted into a small amount
of dental wax (Surgident Periphery Wax) placed at opposite edges of the Petri dish.
Using dark-field illumination and a stereo dissection microscope, small axons
and connective tissue are gently teased away from the giant axon with Vannas-style
iris scissors and No. 5 Dumont forceps, taking care to avoid damaging the membrane
of the giant axon. We recommend starting proximal to the stellate ganglion and pro-
ceeding distally. Particular caution is needed to avoid cutting small collateral
branches that extend from the giant axon occasionally. They may be detectable as
slight protrusions on the axon surface and the giant axon may be slight reduced distal
to the branch. These collateral branches need to be cut at least 0.1 mm away from the
giant axon. After removal of extraneous tissues, the axon is inspected for the pres-
ence of small holes, which can be identified as white patches due to the influx of
Ca
2
þ
from the seawater. Axons with significant white patches should be discarded
as the Ca
2
þ
activates proteases and may disrupt axoplasmic organization.
Axons to be extruded are removed from the seawater and rinsed briefly in a suitable
intracellular buffer (buffer X, see below). Holding the axon by the distal thread, the
axon is placed briefly on the filter paper to remove excess fluid and cut adjacent to
the proximal thread. The axon is placed on a glass slide or 0 thickness 24
m
60-mm
coverslip to extrude for assay of vesicle motility or many biochemical and radiolabel-
ing studies (
Brady et al., 1985, 1993; Leopold et al., 1994
). To extrude, a section of
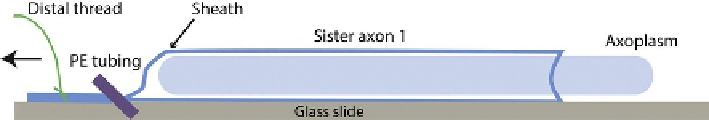
PE190 tubing is used to compress the distal gently but firmly and the axon is pulled
using the distal thread. This leaves a cylinder of axoplasm behind on the coverslip
(
Fig. 9.3
). Typically, a cylinder of 2-2.5 cm in length and roughly 0.4 mm in diameter
FIGURE 9.3
Extrusion of axoplasm. Once the axon is dissected and cleaned of surrounding small axons/
connective tissue, the axoplasmmust be extruded. Briefly, the axon is handled by the threads
and rinsed in intracellular buffer (buffer X) to remove Ca
2þ
containing sea water. The axon is
then blotted on filter paper and cut adjacent to the proximal thread. Using the distal thread,
the axon is placed on the coverslip or glass slide. If the goal is to extrude an axoplasm on
the surface, one places pressure on the axon close to the thread with a short piece of PE190
tubing and pulls the axon with the thread holding the tubing in place. To extrude into a
buffer chamber, the axon is held stationary and the tubing is moved toward the proximal end.