Biomedical Engineering Reference
In-Depth Information
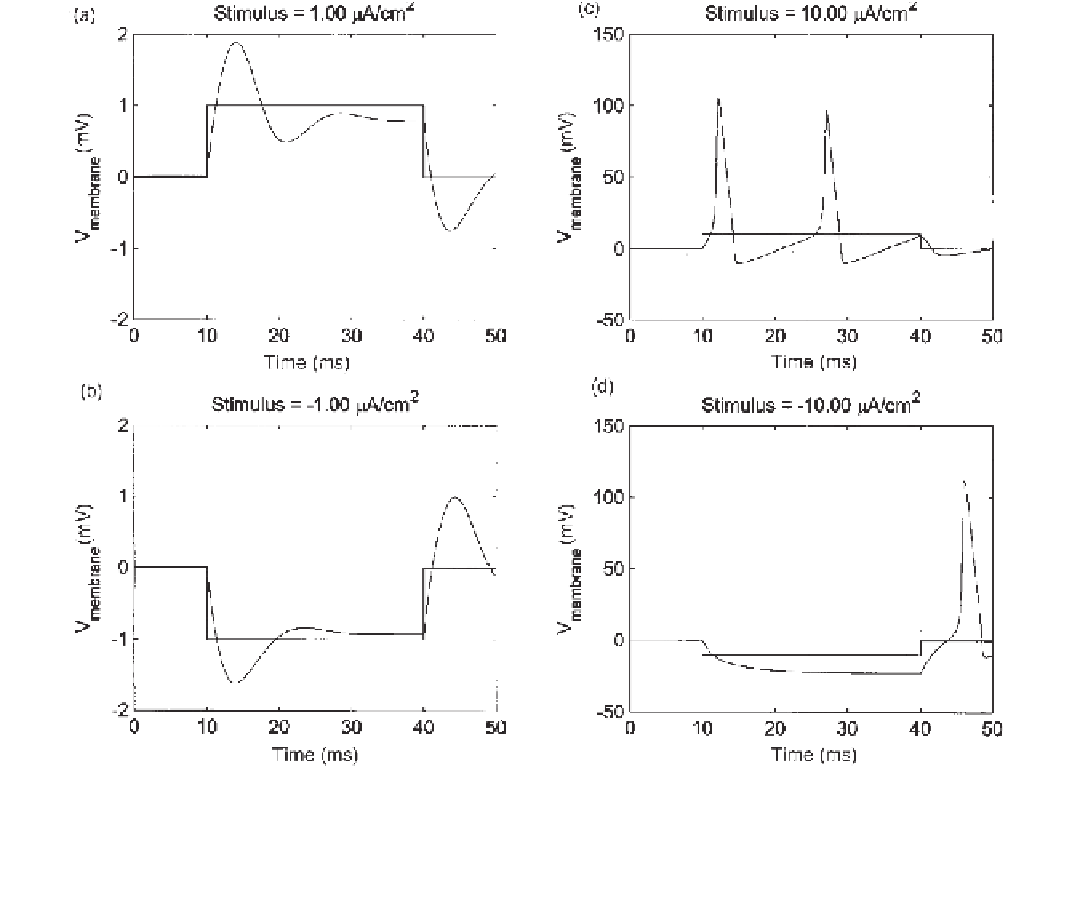
Figure 7.2
Simulation results from the stimulation of a giant squid axon with weak (1 µA/cm
2
) and strong (10 µA/cm
2
) depolarizing (pos-
itive electrode inside the cell) and hyperpolarizing (negative electrode inside the cell) stimuli. (
a
) and (
b
) The change in membrane voltage
evoked by the weak stimuli is related primarily to the change in charge across the membrane's capacitance. (
c
) A strong depolarizing stim-
ulus (
10 µA/cm
2
) takes the membrane voltage over the threshold, causing action potentials for the duration of the stimulus. (
d
) A strong
hyperpolarizing stimulus (
10 µA/cm
2
) yields an action potential at the trailing edge of the pulse through rebound excitation.
capacitance. However, the strong depolarizing stimulus (
A/cm
2
) takes the membrane
voltage over threshold, causing action potentials for the duration of the stimulus.
The strong hyperpolarizing stimulus (
10
µ
A/cm
2
) also yields an action potential, but
only at the trailing edge of the hyperpolarizing pulse. This is what Hodgkin and Huxley
referred to as anode break excitation or
rebound excitation
. The hyperpolarizing pulse
decreases the potassium conductance and removes sodium inactivation. The former leads
to less hyperpolarizing current and the latter to more depolarizing current. Since the kinet-
ics of the potassium channels are slower than that for the sodium channel gate, a transient
depolarization takes place after a prolonged hyperpolarizing voltage, which if large
enough can generate an action potential.
What we would like you to remember as we move to discuss the clinical uses of elec-
trical stimulation is that anodic currents are usually responsible for the activation of
excitable tissue
when the current is delivered through an intracellular electrode
. In addi-
tion, we would ask you to remember that hyperpolarizing currents can also lead to activa-
tion of excitable tissue via the rebound excitation mechanism.
10
µ


Search WWH ::

Custom Search