Biomedical Engineering Reference
In-Depth Information
FIGURE 2.29
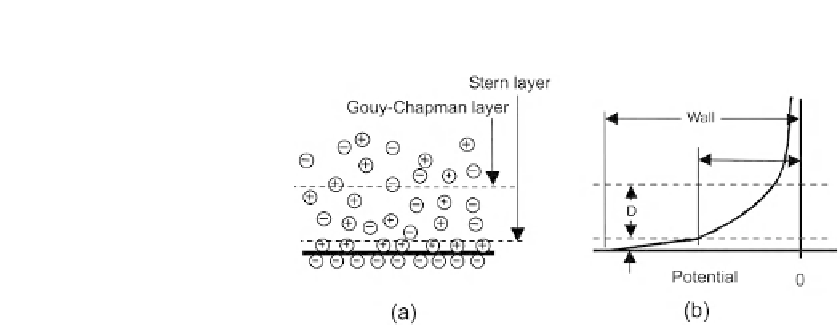
The Debye layer: (a) the Stern layer and the Gouy-Chapman layer and (b) the potential distribution near the wall.
form a thin charge layer, which is called the Stern layer. The Stern layer is attracted to the surface
due to the electrostatic force. The layer leads to the formation of a thicker charge layer in the
solution. This diffuse and mobile layer is called the Gouy-Chapman layer. The Stern layer and the
Gouy-Chapman layer together form the Debye layer (
Fig. 2.29
(a)). Because the Gouy-Chapman
layer is mobile, it can move if an electric field is applied. The interface between the Stern layer and
the Gouy-Chapman layer is called the shear surface. The potential of the charged solid surface is
called the wall potential
J
wall
. The potential of the shear surface is called the zeta potential
(
Fig. 2.29
(b)). The potential distribution in the electrolyte solution can be described by the one-
dimensional Poisson equation:
d
2
J
d
y
2
¼
r
el
ð
y
Þ
;
(2.127)
3
where
r
el
and
3
3
0
3
r
are the electric charge density and the dielectric constant of the electrolyte,
respectively. Assuming the Boltzmann distribution for the charge density, the ion concentration in the
electrolyte solution can be determined as:
¼
n
iN
exp
z
i
e
J
k
B
T
n
i
¼
;
(2.128)
is the ion concentration of the electrolyte with a unit of 1/m
3
,
z
i
is the ionic valence, and
where
n
iN
10
19
is the elementary charge. Thus, the total charge in the double layer is:
e
¼
1,602
N
r
el
¼
n
i
z
i
e:
(2.129)
i
The charge density
r
el
in a symmetric electrolyte is proportional to the concentration difference
between cations and anions:
r
el
w
ze
ð
n
þ
n
Þ
2
zen
N
sinh
ze
(2.130)
r
el
¼
k
B
T
J
:





Search WWH ::

Custom Search