Biomedical Engineering Reference
In-Depth Information
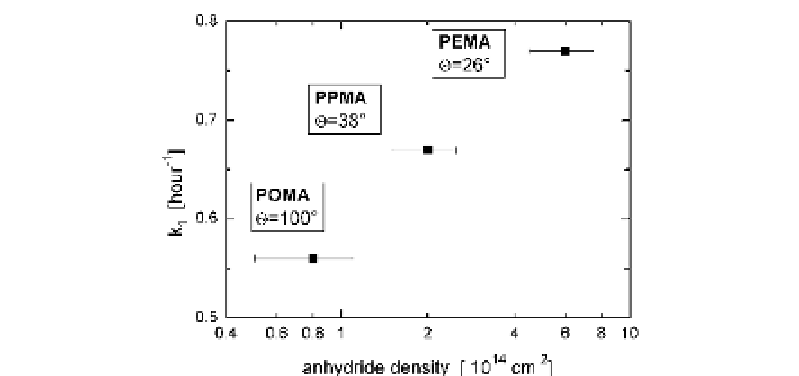
Fig. 1
Fibronectin anchorage strength in terms of time constant of double exponential
fibronectin displacement kinetics (
Γ
2
exp[- k
2
t]) on different maleic
anhydride copolymer surfaces characterized by the density of anhydride functionalities
and water contact angle (poly(octadecene-
alt
-maleic anhydride)—POMA, poly(propene-
alt
-maleic anhydride)—PPMA, poly(ethylene-
alt
-maleic anhydride)—PEMA)
Γ
=
Γ
1
exp[- k
1
t] +
fibril pattern together with the focal adhesion density and a correlation of
these features to the variation in fibronectin substrate anchorage. As shown
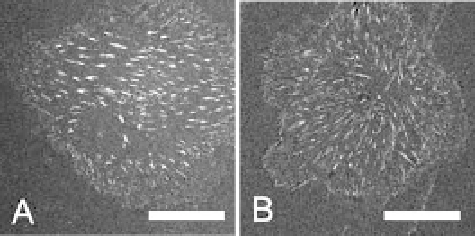
in Fig. 2 endothelial cells can reorganize rhodamine-conjugated fibronectin
to a much greater extent on the hydrophilic poly(ethylene-
alt
-maleic anhy-
dride) substrate. Furthermore, the mean distance between the fibronectin
fibrils was found to be smaller on those substrates. Together with the analysis
on other copolymer substrates the fibril spacing could be directly correlated
to the fibronectin anchorage strength—characterized by the time constant of
fibronectin heteroexchange—as is shown in Fig. 3.
Together with an analysis of the focal adhesion pattern the following work-
ing model could be established from these results demonstrating the impact
of the substrate physicochemistry on fibronectin fibrillogenesis. The overall
Fig. 2
Pattern of fibronectin fibrils after 50 minutes of reorganization by endothelial
cells on poly(octadecene-
alt
-maleic anhydride) (
A
) and poly(ethylene-
alt
-maleic anhy-
dride) (
B
).
Scale bar
: 20
µ
m