Biomedical Engineering Reference
In-Depth Information
The homogeneity and size of the supramolecular assembly were sequence-
sensitive: peptides of the same length behaved differently when they had
different polar head or hydrophobic tail sequences. Such phenomena have been
described theoretically and experimentally in other amphiphilic systems. The
shapeandsizeoftheassembliesareultimatelydependentonthesizeandgeom-
etry of their constituents [71]. In order to visualize the structures in solution, we
utilized the transmission electron microscope with the quick-freeze/deep-etch
method for sample preparation [72], to preserve the structures that formed in
solution for electron microscopy. We observed discrete nanotubes and vesicles
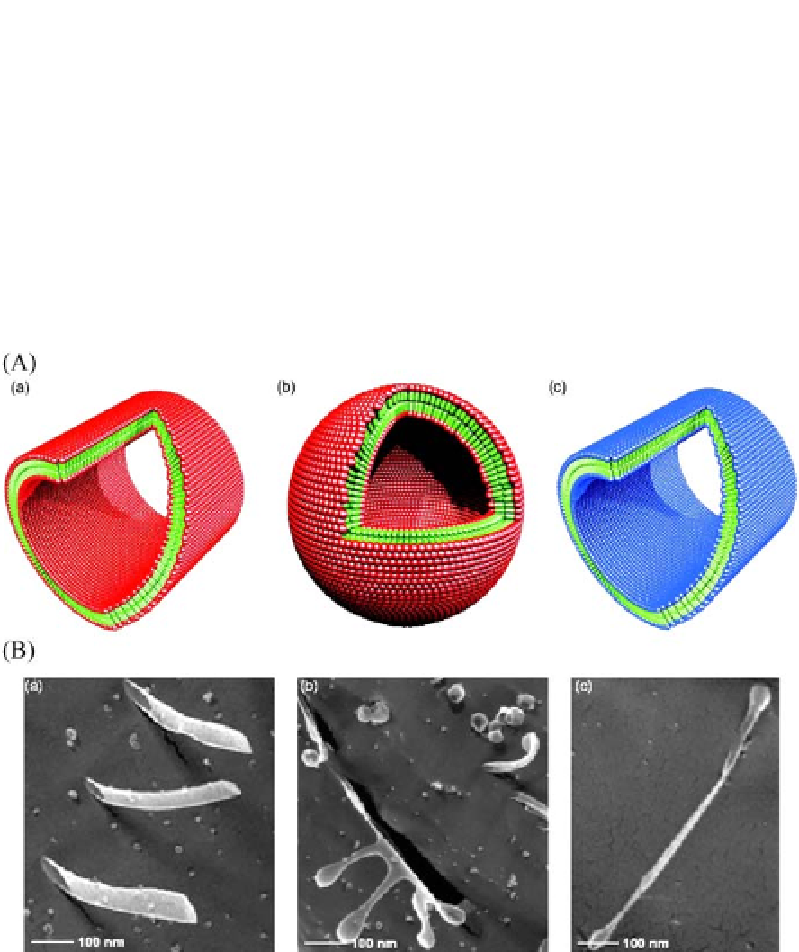
Fig. 5 A
Molecular models of surfactant peptides V6D and K2V6. These peptides have hy-
drophilic heads; either negatively charged aspartic acid or positively charged lysine with
hydrophobic valine tails [13-15].
a
V6D in nanotube form. Billions of these molecules
self-assemble to sequester the valine tails from water in
b
vesicle form or
c
nanotube
form with positively charged heads. These nanostructures are rather dynamic undergo-
ing assembly and disassembly. Color code: green-hydrophobic tails, red-aspartic acid,
and blue-lysine.
B
Quick-freeze/deep-etch transmission electron micrographs of struc-
tures from surfactant peptides.
a
The nanotubes are clearly represented, with a diameter
∼
30-50 nm.
b
The nanotubes and vesicles are visible in the same frame suggesting that
these structures are quite dynamic. It is plausible that the vesicles may be budded out
from the nanotubes and/or they may fuse to form nanotubes in a reversible manner [13-
15]. The diameter of these nanostructures is
∼
30-50 nm.
c
Phosphor-serine surfactant
peptides form nano Q-tips