Biomedical Engineering Reference
In-Depth Information
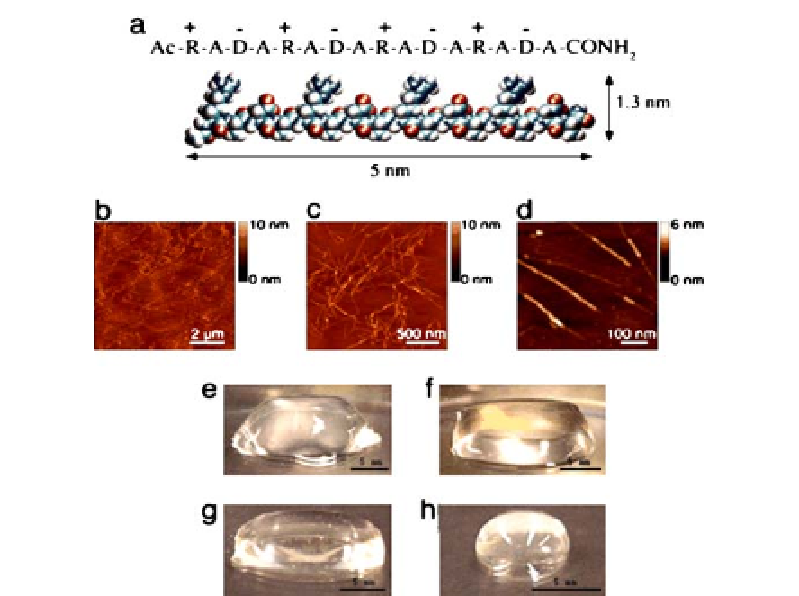
Fig. 4
Peptide RADA16-I.
a
Amino acid sequence and molecular model of RADA16-I. The
dimensions are 5 nm long, 1.3 nm wide, and 0.8 nm thick;
b
-
d
AFM images of RADA16-I
nanofiber scaffold. Note the different height of the nanofiber,
1.3 nm in
d
,suggesting
adouble-layerstructure.
e
-
h
Photographs of RADA16-I hydrogel at various conditions:
0.5 wt% (pH 7.5) in
e
, 0.1 wt% (pH 7.5, Tris.HCl) in
f
, 0.1 wt% (pH 7.5, PBS) in
g
before
sonication, and reassembled RADA16-I hydrogel after four rounds of sonication in
h
.
≈
of extracellular matrices, allowing cells to reside and migrate in a 3D environ-
ment, and molecules, such as growth factors and nutrients, to diffuse in and
out very slowly. These peptide scaffolds have been used for 3D cell culture,
controlled cell differentiation, tissue engineering and regenerative medicine
applications [59, 60].
3.1.1
Nanofibrils from
α
-helices
Several laboratories have designed fibrillar structures based on coiled-coil
structural motifs, ranging from two-stranded to five-stranded coiled-coil
structures [61-65]. In each case, investigators have recognized that peptides
containing the coiled-coil motifs can self-assemble into a staggered interaction
structure. Electrostatic interactions favor the formation of staggered arrange-
ments of helices by two different 28-residue peptides. To help stabilize the
staggered interactions, Woolfson's laboratory also took advantage of a buried
asparagine residue in each of the two peptides, which can form structure sta-