Biomedical Engineering Reference
In-Depth Information
To provide tissue-mimetic environments for adherent cells glycosamino-
glycans such as heparin and hyaluronic acid were implemented into three-
dimensional collagen gels during self-assembly of monomeric collagen [8].
Turbidity measurements were utilized to follow the formation of collagen fib-
rils in the presence of heparin and hyaluronic acid that was initiated by an
increase in temperature, pH value, and electrolyte content of a cold acidic
solution of collagen monomers in the bulk volume. Varied optical densities
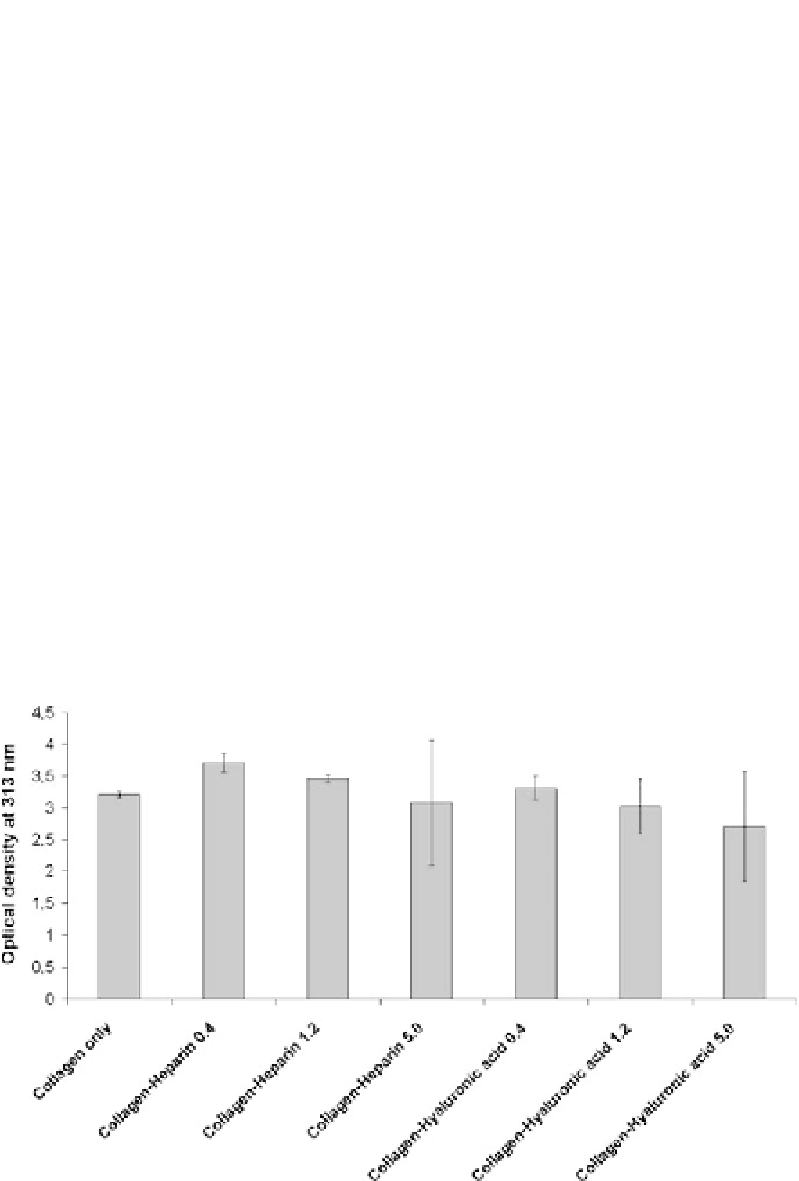
were observed for different concentrations of glycosaminoglycans in com-
parison to pure collagen (Fig. 6). Gradually increasing portions of heparin
and hyaluronic acid, respectively, at constant collagen concentrations caused
a slight decline in the maximum optical densities indicating that fibril forma-
tion was obviously affected by the presence of the glycosaminoglycans. The
differences in turbidity values were concluded to be either caused by different
quantities of collagen fibrils or varying fibril diameters.
To covalently immobilize collagen and its assemblies with glycosamino-
glycans, fibrillogenesis was performed in the presence of polymer-coated
substrates resulting in thin layers of collagen fibrils. Therefore, cold mixtures
of dissolved collagen and heparin or hyaluronic acid at different concentra-
tions were exposed to glass slides or silicon wafers which had been modified
before with thin films of poly(octadecen-
alt
-maleic anhydride) followed by
the initiation of fibrillogenesis through an increase in temperature. The re-
Fig. 6
Turbidity measurements of collagen fibrillogenesis in the presence of heparin and
hyaluronic acid 2 h after initiation of fibril formation. Initial concentration of the non-
fibrillar collagen solution was 1.2 mg
/
ml. Concentrations of the glycosaminoglycans were
0.4, 1.2, and 4.0 mg
/
ml, respectively