Biomedical Engineering Reference
In-Depth Information
Electrochemical deposition is frequently employed for growing not only thin
films but also nanowires. The deposition procedure is restricted to a template
consisting of nanopores, which is coated with a metallic thin film acting as a cathode
on one side. Semiconductor nanowires of CdS, metallic nanowires of Co, Fe, Cu,
Ag, Au, Pb, Ni, or superlattices A/B containing two constituents A and B, for
instance, Co and Cu, were fabricated using the electrochemical deposition.
Extremely thin nanowires can be grown using MOCVD or CVD. In these
methods, the precursor material of the nanowire is heated to generate vapors that
fill the nanopores of the template, which is subsequently cooled to obtain the solid
nanowires. Almost single-crystal nanowires are fabricated with the CVD method,
polycrystalline nanowires being obtained otherwise. Examples of single-crystal
nanowires grown with CVD techniques include GaAs, GaN, Bi, and InAs, with
diameters smaller than 10 nm, as well as CNTs. A recent review of carbon growth
techniques and applications in the area of electronics is
Javey and Kong
(
2009
).
The vapor-liquid-solid (VLS) growth method of nanowire relies on the fact that
a liquid (L) droplet of a catalyst absorbs the vapors (V) of the source material.
The nanowire forms as a result of the solidification (S) of the source material
due to the liquid saturation and a subsequent nucleation process, which produces
a preferential site for future deposition at the boundary of the liquid. Thus, other
nucleation processes are avoided, and growth is allowed only in a single direction,
as displayed in Fig.
1.27
. The VLS method has been used to grow nanowires of Ge,
Si, and ZnO. In situ electron microscopy can visualize and control the growth of
Si and Ge NWs through the VLS method. Images and movies are produced in a
transmission electron microscope, which is able to deposit catalysts and introduce
CVD precursor gases in the sample under observation. In this way, the nucleation,
the surface structure, and the growth kinetics are measured (
Ross 2010
).
Nanowires could be grown to form superlattices of the form XYXYXYXY. . .
consisting of two types of nanowires, X and Y (
Chik and Xu 2004
). Figure
1.28
represents an InP/InAs superlattice (
Bj ork et al. 2002
), while Fig.
1.29
illustrates
the energy band of a superlattice made of an alternation of intrinsic CNTs and
vapors (V)
liquid
catalyst (L)
growth
direction
nanowire (S)
Fig. 1.27
The VLS method of growing nanowires



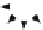































Search WWH ::

Custom Search