Biomedical Engineering Reference
In-Depth Information
Fig. 2.43
Graphene Hall
constriction (done by oxygen
plasma etching) acting as a
gas sensor
Au/Ti contact
graphene
Si/SiO
2
organic compounds of lung cancer using a random network of functionalized CNTs
drop cast on interdigitated electrodes (
Zilberman et al. 2010
).
The ultimate detection limit could be obtained using graphene gas sensors.
Graphene gas sensors are able to detect even a single molecule of gas attached or
detached from the graphene surface (
Schedin et al. 2007
). The changes in graphene
resistivity are about
C
4%forNH
3
;
4%forNO
2
,or
1% for H
2
O after 200 s, all
gases having a concentration of 1 ppm.
The graphene sensor has the shape of a Hall bar (see Fig.
2.43
), and thus, Hall
measurements have shown that NH
3
, CO, and ethanol are donors, and NO
2
,H
2
O,
and iodine are acceptors. Although chemical doping induces impurities in graphene,
these are kept at a relative low level, since no significant changes of mobilities were
observed even beyond carrier densities greater than 10
12
cm
2
. The detection limit
of this sensor is 1 ppb, meaning a variation of graphene resistivity of =
/
10
4
.
Using few graphene layers to decrease the contact resistance around 100,the
changes in adsorption and desorption of a single NO
2
molecule were monitored
using Hall resistivity; a difference in the resistance of about 2:5 was recorded
each time when an electron was adsorbed or detached from graphene. In this way,
the detection sensitivity is of only a single molecule, but this implies complicated
measurement procedures. In reality, for normal electronic noses, sensitivities of
few parts per billion are easily obtained with a sensor made from a graphene
monolayer with two metallic contacts, in which current flow is monitored. The
functionalization of graphene with various sequences of ssDNA enables sequence-
dependent detection and chemical recognition capability; hundreds of distinct
sensor responses can thus be engineered, and electronic nose behavior becomes
possible (
Lu et al. 2010
).
Lung cancer can be detected in exhaled breath using volatile organic compounds
(VOCs), which in healthy breath are in concentrations of 1-20 ppb, while in the case
of cancer, their concentrations increase up to 100 ppb. In fact, there are 42 VOCs
used as markers (
Peng et al. 2009
). An array of nine cross-reactive chemiresistors
is used to detect a variety of odorants, which are relevant for lung cancer. The
chemiresistor is based on assemblies of 5-nm gold nanoparticles functionalized with
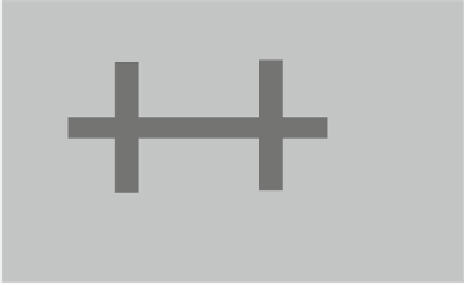


















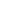

























Search WWH ::

Custom Search