Biomedical Engineering Reference
In-Depth Information
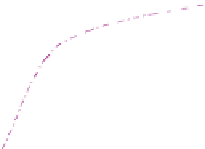
0.006
System: hydrogen and water
0.005
0.004
298 K
323 K
348 K
373 K
0.003
0.002
0.001
0
0
100
200
300
400
500
Pressure (atm)
FIGURE 9.13
Hydrogen solubility in water. Source: From Ji et al. (2006).
0.035
System: carbon dioxide and water
0.03
0.025
0.02
298 K
323 K
348 K
373 K
0.015
0.01
0.005
0
0
100
200
300
400
500
Pressure (atm)
FIGURE 9.14
Solubility of carbon dioxide in water. Source: From Ji et al. (2006).
The liquid mixture is next depressurized through a pressure regulator
before it enters the second separator (S2,
Figure 9.15
). The solubility of most
gases reduces with a decrease in pressure, so the second unit separates the
rest of the CO
2
from the gas.
Feng et al. (2004a,b) calculated the phase equilibrium of different gases
in water for a plant using different relations. Values calculated using SAFT
equilibrium showed the best agreement with experimental results. These


































































































Search WWH ::

Custom Search