Biomedical Engineering Reference
In-Depth Information
9.3.2 Hydrolysis
Hydrolysis (meaning “splitting with water”) is the reaction of an organic
compound with water. Here, a bond of an organic molecule is broken, and
the water molecule is also broken into [H
1
] and [OH
2
]. The organic mole-
cules are cleaved into two parts by the water molecule: One part gains the
[H
1
] ion; the other part, the [OH
2
] ion. Acid or base catalysts generally cat-
alyze hydrolysis reactions. Water near its critical point (at high temperature
and pressure) has a high ion product, so the water itself catalyzes the hydro-
lysis reaction.
A simplified representation of the reaction scheme is shown in
Figure 9.5B
with polyethylene terephthalate (PET) as an example. The hydrolysis of PET
into terephthalic acid and ethylene glycol in SCW is a better option than other
reactions (e.g., methanolysis or glycololysis) because it does not require sol-
vents and catalysts like others. Here, water near its critical point is used to
accomplish this reaction in a shorter time. Additionally, SCW avoids the need
to recover and dispose of external solvents or catalysts.
Figure 9.5A
shows the
(A)
(B)
Pet(polyethylene terephthalate)
H
2
O
2
O
H
2
OH
2
O
OC
O
C
CH
2
OC
O
C
O
O
CH
2
CH
2
2
O
Pet(polyethylene terephthalate)
Hydrolysis
HO C
O
C
OH+HO
CH
2
CH
2
OH+HO
C
C
O
OH+HO
CH
2
CH
2
OH
O
O
Ethylene glycol
Terephthalic acid
Ethylene glycol
Terephthalic acid
FIGURE 9.5
(A) The photograph shows PET in SCW and SCW after hydrolysis.
(B) Hydrolysis of PET in SCW. Source: From Kobe Steel (2010).













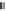


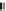





















Search WWH ::

Custom Search