Biomedical Engineering Reference
In-Depth Information
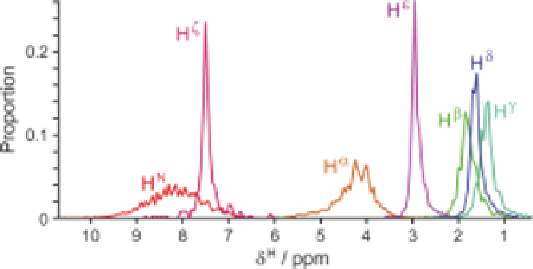
Distributions of
1
H chemical shifts observed for lysine residues in the
RefDB database,
19
adapted from the Reference Chemical Shifts tool in
the CCPN Analysis program.
94
Figure 3.2
them choose the most appropriate profile for each nucleus. More rigorous
statistical analyses are used in automated systems that check user-selected
assignments, determining probability scores that can highlight unlikely
choices. The Assignment Validation Software (AVS) suite of tools determines
a Bayesian posterior probability for each assignment in a new BMRB
submission, so that obvious outliers can be queried.
25
Despite their usefulness,
one-dimensional (1D) chemical shift distributions alone are not sufficiently
discriminating to resolve all assignment problems and are typically supple-
mented with additional information about the spin system and neighbouring
residues.
27,38-41
3.4 Reference States and Secondary Chemical Shifts
In order to quantify chemical shift changes that are induced by protein
structure, it is important to define a reference state that can indicate the
resonance frequencies expected for nuclei in different amino acid residues when
no structural features are present. The chemical shifts measured in an ideal
'random coil' state would result from fast exchange among the energy-
weighted populations of all sterically allowed polypeptide conformations, in
the absence of long-range inter-residue interactions.
42
Observed (d
ob
i
)and
random coil (d
r
i
) chemical shifts can be used to calculate secondary chemical
shift values (Dd
i
) for each site i,
Dd
i
~d
ob
i
{d
rc
,
ð
3
:
5
Þ
i
which should capture all of the effects of secondary, tertiary and quaternary
structure that remain once the primary sequence of the polypeptide chain has
been taken into account.
43,44
Under highly denaturing conditions, the unfolded form of a protein can
provide an ideal intrinsic reference state, allowing secondary shifts to be