Biomedical Engineering Reference
In-Depth Information
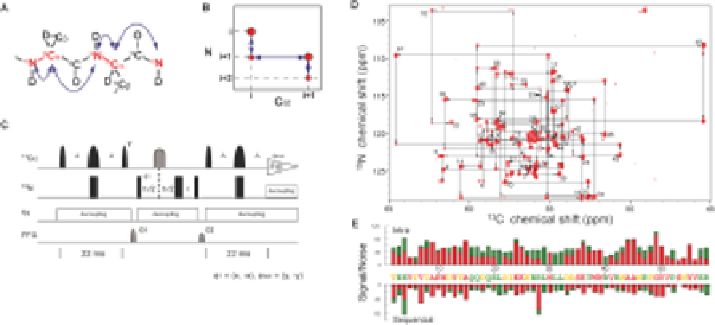
Figure 2.3
Two-dimensional (2D) NCA HSQC experiment using alternate
13
C-
12
C-
labelled samples. (A) Correlations observed in a 2D NCA HSQC
experiment using alternate
13
C-
12
C-labelled samples. (B) Schematic
representation of a 2D NCA HSQC spectrum. Each
13
C-labelled carbon
yields two signals corresponding to C
a
i
-N
i
and C
a
i
-N
i+1
correlations,
which allow sequential linking of the backbone nuclei. (C) Pulse program
of the NCA HSQC experiment optimized for glycerol/D
2
O labelling.
Note that no J
ab
constant time or C
a
selective pulses are used in the pulse
schemes. These are only necessary for uniformly labelled samples, which
need additional delays and causes imperfect magnetisation transfer for
certain residues. (D, E) Sequential main-chain assignments in a NCA
HSQC experiment using C
a
direct detection. (D) NCA HSQC spectrum
recorded for 1 mM [ul-
2
H
15
N, [2-
13
C] glycerol] Nck SH3.1. Arrows
indicate the sequential walk. Note the proline
15
N signal (residue 52) is
folded from its low-field position. (E) S/N for intra-residue (upper) and
sequential (lower) cross peaks. Red and green bars are for samples
labelled with [2-
13
C] and [1,3-
13
C] glycerol, respectively. Adapted from
ref. 50, where details of the experimental conditions can be found.
15
N
H
(Figure 2.1).
resonances relax much slower than deuterium-attached
Thus selecting this component may be beneficial.
As shown in Figure 2.3(D), the larger dispersion of
13
C with respect to
1
H
enabled unambiguous assignment for most of the sites in a single experiment. As
expected, the intensities of the observed correlations largely depend on the
13
C
a
-
labelling rate. In total, however, the information from both 2-
13
C and 1,3-
13
C
glycerol-labelling schemes combined with deuteration established all intra- and
sequential correlations from the structured region of the protein including a
proline, which is not accessible with
1
H
N
-detected experiments [(Figure 2.3(E)].
On the other hand, for the protonated protein, only 87% of sequential
correlations are observed, indicating that full deuteration is a key factor for the
C
a
-detection experiment. It is worth noting that a single correlation that isnot
observable for an exchange-broadened residue (Asn 54) in a regular
1
H-
15
N
HSQC was successfully assigned for both N and C
a
resonances based on the
NCA correlations. This indicates the clear advantage of C
a
detection over
proton detection experiments for an exchange-broadened site.