Biomedical Engineering Reference
In-Depth Information
when studying these samples is usually the fact that the quantity of labelled
ligand is very low (often below 1 mg) and that large amounts of lipid and
receptor are present in the sample. This not only affects the sensitivity, but also
means that natural-abundance
13
C signals from the receptor and lipid are
observed in the spectra. This latter issue has been addressed by recording
double-quantum spectra.
34,35
The fact that solid-state NMR is insensitive to
the size of the assembly studied has enabled the investigation of several large
systems
such
as
the
phage
coat
protein
in
hydrated
infectious
Pf1
bacteriophage
38
or HIV-1 capsid protein assemblies.
39
A further class of proteins, which is well suited to solid-state NMR and
which are difficult to study at high resolution by any other means, are
cytoskeleton binding proteins. Initial studies of actin- or microtubule-bound
proteins have been conducted,
40,41
although no high-resolution structural
information has been reported to date. However, for the myelin basic protein
(MBP) interacting with model membranes, the helical interactions with lipid
bilayers (Figure 13.2) was refined using specific labelling of fragments of
MBP.
40
Finally, there are several other heterogeneous systems that are inaccessible
to many other techniques but that have been successfully studied using solid-
state MAS NMR. aB-crystallin, for example, which forms polydisperse
oligomers was investigated as a PEG precipitate and a structure of the dimer
unit that makes up the large oligomers was determined.
42
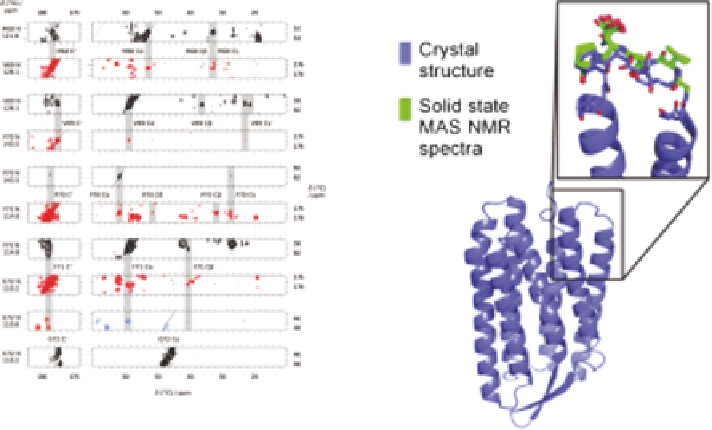
Heterogeneous
Solid-state MAS NMR spectra (left) of U-
13
C,
15
N 2D crystalline
bacteriorhodopsin used for assignment of Met68-Gly72 (loop A-B)
using NCACX, NCOCX (black), CANCO (orange), and CAN(CO)CX
(blue) at various mixing times, to determine the conformation of the A-
B loop in natural purple membranes. Adapted from ref. 30.
Figure 13.2