Biomedical Engineering Reference
In-Depth Information
lography (Figure 13.1)
22,30
has been used to assign loops in bacteriorhodopsin
in natural (purple) membranes.
30
Here, comparison of structural constraints
for (less well-resolved in X-ray diffraction) loops, shows the possible
perturbations that may be induced upon crystallisation. Some 18 or more
structures of bacteriorhodopsin exist, but here complementarity between
NMR and diffraction information might add to the structural models.
Although there is no intrinsic size limit to the proteins studied by solid-state
NMR in the way in which there is for solution NMR, the spectral overlap
encountered with large proteins can be intractable. Membrane protein samples
tend to suffer from lower sensitivity than microcrystalline samples which
makes it more difficult to record the multi-dimensional spectra required to
resolve crowded spectral regions. In this regard, the increased resolution
offered by higher magnetic fields is particularly beneficial for solid-state NMR
studies of large membrane proteins.
Closely linked to the study of membrane proteins, is that of receptor-bound
proteins,
31-33
peptides
34,35
and small-molecule ligands.
35-37
The limiting factor
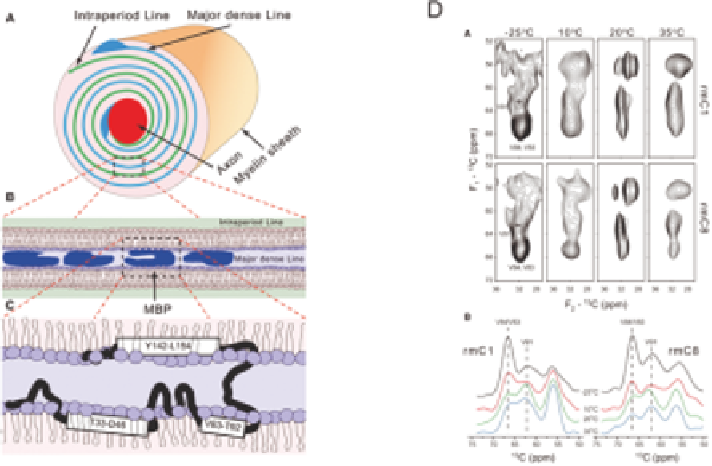
Figure 13.1
Model (A) for the structure of the myelin sheath that consists of stacked
lipid bilayers to which myelin basic protein (B) peripherally binds. There
are three potential amphipathic helices in MBP (C). Solid-state NMR
13
C-
13
C correlation spectra (D; upper, Val C
a
/C
b
; lower, projections
showing redistribution of intensities from the Val83/Val84 C
a
to the
Val91 C
a
region) at different temperatures of specifically labeled (Val)
MBP and a variant (rmC1 and rmC8) interacting with bilayers, were
used to show that the short (Val83-Lys88) helical structure of the
immunodominant epitope of MBP is not sensitive to the overall
electrostatic charge of the protein in the reconstituted system studied.
Adapted from ref. 36.