Biomedical Engineering Reference
In-Depth Information
concentrations above the CMC and the protein-to-detergent ratio adjusted for
best results.
105
As well as being suitable for structural studies, micelles are in many cases
believed to be a crude but frequently satisfying mimic of the cell membrane.
They emulate solubilisation of proteins in the bilayer by covering the
hydrophobic surface of the protein with hydrocarbon tails, whilst the detergent
headgroups remain in contact with the solvent and hydrophilic regions of the
protein. This orientation has been confirmed using protein-detergent NOEs
106
and for pSRII [Figure 12.2(a)] using water and detergent-soluble paramagnetic
reagents.
19
In both cases, the detergent tails cover the expected hydrophobic
surface of the protein. These studies also predict a prolate ellipsoid, rather than
torus-like orientation of detergent around the membrane protein.
A variety of studies have aimed to identify suitable classes of detergents
which fulfil the above criteria for membrane proteins.
96,107-110
Whilst these
studies have typically suggested some general characteristics and identified
classes of detergents that appear particularly favourable under the conditions
examined, it is clear that this process is highly specific to the membrane protein
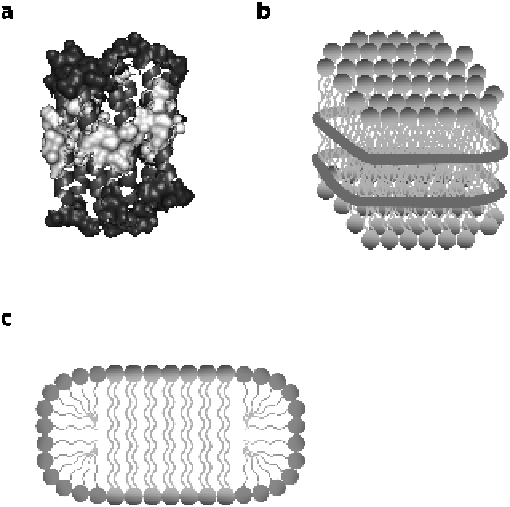
Figure 12.2
Membrane mimics: (a) micelle-solubilised pSRII showing the surface
area interacting with detergent tails (light spheres) and the area
interacting with detergent headgroups (dark grey spheres), determined
using water and detergent soluble paramagnetic reagents (see ref. 19).
(b) Nanolipoprotein particle consisting of a bilayer of lipids surrounded
by two copies of an amphipathic a-helical protein (cylinder). (c)
Schematic of a bicelle, showing the lipid bilayer (long, double-chain
tails), with ends closed by detergent (short, single-chain tails).