Biomedical Engineering Reference
In-Depth Information
as the C-H bond changes orientation relative to the magnetic field, either due
to internal or overall motions; in some orientations the proton field adds to the
external magnetic field, whereas in other orientations it subtracts or hasno
contribution [Figure 9.1(A)]. This angular dependence is described by
S
3 cos
2
h
{
2
T, where h is the angle between the inter-nuclear vector and the
magnetic field, and the angular brackets denote a time-average over all
orientations sampled at a rate faster than the dipolar coupling [Figure 9.1(B)].
Under conditions of random molecular tumbling, the angular term averages
to zero and the proton does not affect the average field at the carbon nucleus;
therefore, the observed carbon frequency is unchanged. As a result, RDCs are
not observable under normal solution conditions. However, by imparting a
small degree of order on the molecule, the angular term no longer averages to
zero, and the carbon nucleus experiences a residual proton field in addition to
the external magnetic field. Since half of the proton nuclei are aligned parallel
and the other half anti-parallel to the field, the proton fields add to the external
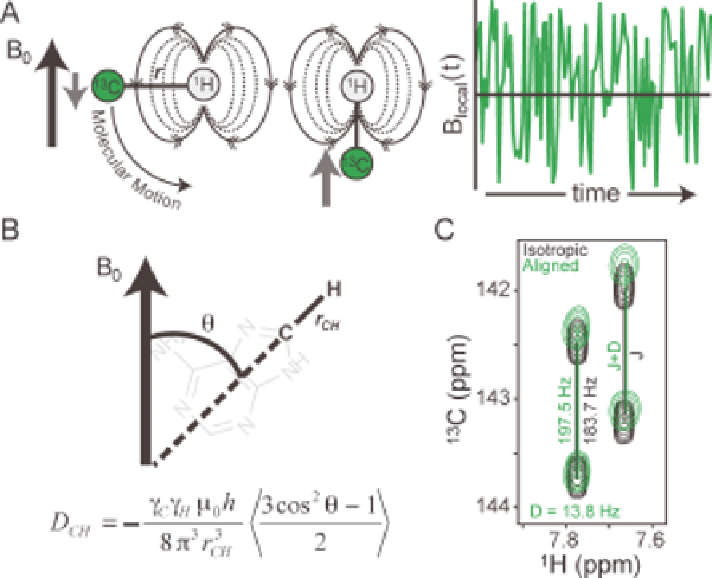
Figure 9.1
Physical origin and measurement of RDCs. (A) The reorientation of bond
vectors leads to an oscillating local magnetic field at the nucleus of
interest. (B) RDCs between spins i and j (C and H, respectively) provide
long-range constraints on the average orientation (h) of the inter-nuclear
bond vector relative to the magnetic field (B
0
). (C) Measurement of RDCs
as new contributions to resonance splittings (black resonances) observed
upon partial alignment (green resonances).