Biomedical Engineering Reference
In-Depth Information
zinc-binding ligands, which may consist of four Cys or two His, two Cys, or
other combinations. The original zinc-finger proteins were of the Cys
2
His
2
type,
78
which consist of a b-hairpin and a short a-helix, held together by the
presence of zinc.
79
Although a single finger can bind DNA, significant
specificity as well as greater affinity requires the presence of multiple fingers;
the minimal number of finger units to promote binding to a specific DNA
sequence appears to be three.
80,81
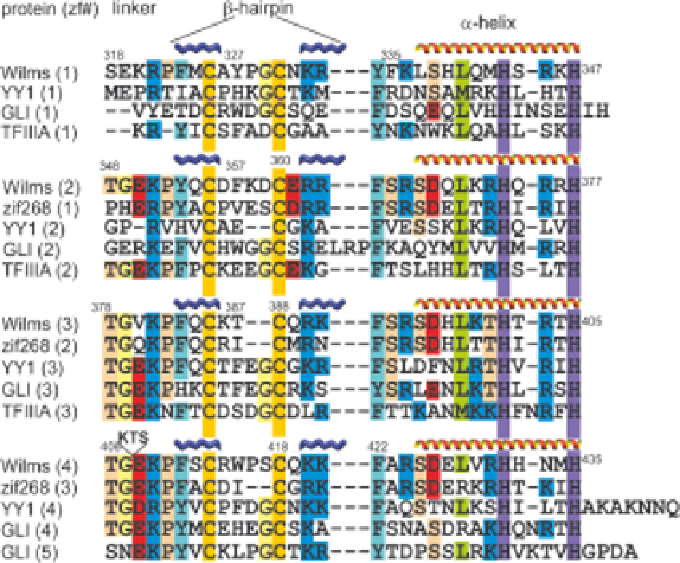
As might be expected, zinc fingers of the same type show considerable
sequence homology (Figure 5.8). Intriguingly, the homology extends to the
five-residue linker sequences between fingers, but only for fingers other than
the first of a series. This observation was explained by NMR studies on several
different types of zinc-finger proteins. Firstly, it was shown by NMR that a
free three-finger protein is only partly folded. Each zinc finger is correctly
folded in the presence of zinc, but the three fingers are semi-independently
mobile, with complex dynamics in the free state that include some inter-finger
contacts.
82
A simple illustration is provided by the plot of
1
H-
15
N NOE for a
zinc-finger protein in the presence and absence of the cognate DNA
(Figure 5.9): in the free protein the linker sequences are mobile, but in the
Figure 5.8
Amino acid sequence alignment for various zinc-finger domains, coloured
according to amino acid type. The alternate splice site where the sequence
KTS is inserted is indicated between residues 408 and 408 of the Wilms
tumor protein. Adapted from ref. 90 with permission. # Elsevier, 2007.