Biomedical Engineering Reference
In-Depth Information
D
D
B
C
A
C
A
B
C'
C'
A'
A'
B'
B'
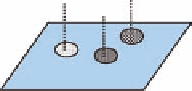
FIGURE 5.5
The three-point receptor theory. Only one enantiomer can form the three correct interactions
with points A¢, B¢, and C¢ on the receptor.
one enantiomer has the optimal spatial disposition of the three groups A, B, and C to interact with the
complementary sites on the receptor. Interactions (possibly more than three) could be ionic, hydro-
phobic, steric, or hydrogen bonding. Although the three-point receptor theory is simplistic, it has
also been used successfully to understand chromatographic resolution of mixtures of enantiomers on
chiral stationary phases (CSPs), which can be thought of as artii cial receptors (see Section 5.5.2).
5.4 WHY IS STEREOCHEMISTRY IMPORTANT IN DRUG DESIGN?
Chiral drugs, sold as single enantiomers, either for an economical or regulatory reason, are likely to
dominate drug markets in the near future. Pharmaceutical companies see enantiomers as a way of
prolonging the patent life of their existing racemic drugs by patenting and then marketing the active
enantiomer thereby undercutting competition from generic drug sales. In addition, some compa-
nies see this switching from racemate to single active enantiomer as a way into the drug market.
However, these are not the only reasons for testing individual enantiomers of chiral drugs. Lessons
learned from mistakes made by marketing racemic drugs also play a part, such as the tragic case
of thalidomide (Figure 5.8). Racemic thalidomide was developed in the 1950s and was used as a
sleeping pill and to treat morning sickness. Unfortunately, the drug had serious side effects as it
was found to be teratogenic causing fetal abnormalities. It was later discovered in tests with mice
that the (
S
)-enantiomer possessed the teratogenic activity while the (
R
)-enantiomer possessed the
sedative activity. However, subsequent studies revealed that the enantiomers racemise under physi-
ological conditions. Despite this, thalidomide brought the role of chirality in drug development into
the spotlight. Recently, thalidomide has hit the headlines again as the use of the racemate for treat-
ment of leprosy has been approved by the Food and Drug Administration (FDA) but only under the
strictest of guidelines. It appears that thalidomide may also have therapeutic utility in the treatment
of AIDS-related disorders and tuberculosis.
The FDA strongly urges companies to evaluate both the racemates and the corresponding indi-
vidual enantiomers as new drugs. Thus, even if a drug is to be sold as a racemate, the individual
enantiomers need to be evaluated, which increases the cost and timescale of drug development.
Therefore, synthesis of single enantiomeric drugs is becoming a priority.
It should be noted that not only the pharmacodynamic aspects are important in the discussion
of the activity of chiral drugs. Pharmacokinetics is also affected as the absorption and clearance of
drugs involves interaction with enzymes and transport proteins. Thus, the individual enantiomers
of a chiral drug may be metabolized by enzymes at different rates and may be transformed into
different chemical entities. As a result of these considerations, it is very important that individual
enantiomers of chiral drugs are tested in the clinic.
Ariëns, a pioneer in the i eld of enantioselective drug actions, has proposed that the active
enantiomer of a chiral drug be termed the eutomer while the less active enantiomer should be
termed the distomer. The eudismic ratio (ER) is dei ned as the ratio of the activity of the eutomer to
that of the distomer. The presence of the distomer in the racemic drug can have a number of conse-
quences for the biological activity.