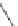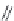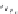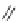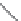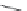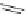Biomedical Engineering Reference
In-Depth Information
O
R
1
O
R
2
O
O
R
1
O O
R
1
OMe
OMe
NO
2
R
2
O
N
R
2
O
O
R
1
OMe
NO
2
Target-oriented synthesis
(a)
Diversity-oriented synthesis
(b)
FIGURE 4.2
(a) Illustration of the principles of TAS and DOS. In TAS the synthesis of a single-target mol-
ecule is convergent, and the synthetic strategy is devised by a retrosynthetic analysis. The synthesis of multiple
target molecules in DOS is divergent and the synthetic strategy planned by forward synthetic analysis. (b)
Example of DOS, where different pairings of the functional groups (ester, alkyne/alkene, and nitro group)
lead to three different scaffolds.
A number of successful examples for the application of DOS for the synthesis of diverse combina-
torial libraries, phenotypic screening, and subsequent elucidation of the biological target have been
carried out and will be discussed in Section 4.2.2. In general, the application of DOS in chemical
and biological studies is mainly carried out by academic laboratories and further generalization of
DOS principles is required to broaden the applicability of DOS in drug design and development.
4.2.1.2 Fragment-Based Approaches
The application of fragments of chemical compounds has been used for some time to simplify
computational analysis of ligand binding and in the analysis of pharmacophore elements. However,
only recently a similar approach has been used in drug screening, the concept being that by screen-
ing low-molecular-weight compounds or fragments, low-afi nity binders are found, and, ideally,
combining or evolving fragments should then lead to chemical compounds with much-improved
afi nity. One of the advantages compared to high-throughput screening (HTS) is that hits from
fragment-based screening generally have lower molecular weight and more efi cient binding afi nity
and provides better starting points for lead generation.
A requirement for fragment-based screening is that a sufi ciently sensitive technology for screening
is available, that is, a technology that allows identii cation of low-afi nity (in the mM range) binders.
In addition, structural information is required to move from binding fragments to high-potent lead
compounds. Therefore screening is carried out using bioassays such as surface plasmon resonance
(SPR) in combination with biophysical techniques such as nuclear magnetic resonance (NMR) tech-
nologies or x-ray crystallography, the latter providing structural guidance for further development.
Once one or more fragments have been identii ed, iterative cycles of medicinal chemistry, ideally
guided by three-dimensional structural data, are performed to optimize these weak binding frag-
ment hits into potent and selective lead compounds. There are principally, two different approaches
for optimization: (1) fragment linking or (2) fragment evolution (Figure 4.3). If two fragments have
been identii ed that bind in separate binding sites that are sufi ciently close to each other, those
fragments can be chemically linked and provide a lead, this is called fragment linking (Figure 4.3).