Biomedical Engineering Reference
In-Depth Information
O
O
NH
O
CF
3
3.14
K
i
= 122 nM
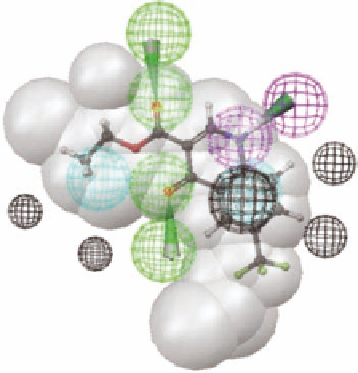
FIGURE 3.8
The most active hit from the database search and its i t to the pharmacophore model.
i tted to the pharmacophore model with its ethyl group corresponding to the fused benzene ring in
the l avones and the CF
3
group corresponding to the isopropyl ester in
3.4
(Figure 3.2). The struc-
ture of the compound is structurally much different from that of l avones and it is a novel compound
in a medicinal chemistry sense as it represents a class of compounds that has not previously been
tested for afi nity for the BZD site of the GABA
A
receptor.
3.5.1 P
OSTPROCESSING
OF
D
ATABASE
H
ITS
—A
N
E
SSENTIAL
R
EQUIREMENT
To avoid false positives, i.e., compounds that are ranked high in a database search, but are found
to be of low afi nity when tested, postprocessing of database hits is, in general, necessary before
selection of compounds for synthesis or purchase. As an example, the second best hit in the data-
base search is compound
3.15
i tting to the pharmacophore model with the hydroxyl group as a
hydrogen-bond donor as shown in Figure 3.9. Even though the compound displays a good i t to the
OH
OMe
3.15
K
i
= 6 400 nM
OMe
OMe
Global energy minimum
0.0 kJ/mol
Bioactive conformation
+48 kJ/mol
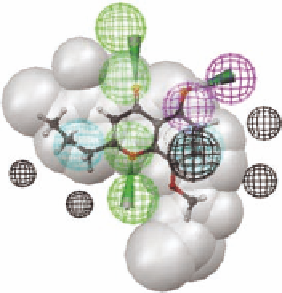
FIGURE 3.9
Compound
3.15
, a top ranking hit in the database search together with the calculated global
energy minimum and the bioactive conformations.