Biomedical Engineering Reference
In-Depth Information
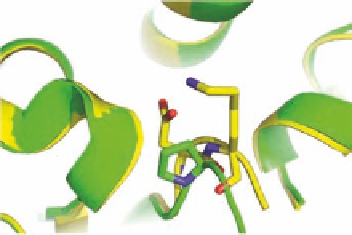
LysB29
AspB28
ProB28
*
*
*
*
(A)
(B)
FIGURE 2.14
(A) Human insulin (pdb-code 1ZNJ, green) and insulin aspart (pdb-code 1ZEG, yellow),
showing the change in conformation of the C-terminus (marked by an asterisk) by the mutation ProB28Asp.
(B) For human insulin ProB28 and for insulin aspart AspB28 and the neighboring LysB29 are shown as stick
models. Heteroatoms are colored red and blue for oxygen and nitrogen, respectively.
beta-sheet and at the same time burying the nonpolar side chains. Removal of the B24-B30 residues
yields monomeric insulin and eliminates the association of monomers into dimers and hexamers.
Furthermore, molecular modeling studies showed that ProB28 was important for dimer formation,
but apparently not involved in receptor binding. Mutation of B28 from Pro to Lys or Asp has a pro-
found effect on dimerization. The ProB28Asp mutant is presently marketed as rapid-acting insulin
named aspart (Novorapid).
The knowledge of the interactions, which stabilize the hexamer and dimer, has made it possible
to engineer the properties of insulin to yield therapeutic insulin analogs with tailored properties.
Homology modeling indicated that inversion of the residues B28 and B29 should give faster act-
ing insulins (Figure 2.15). The double mutant, ProB28Lys + LysB29Pro, indeed had a faster onset
than normal insulin and it was the i rst genetically engineered insulin analog to become available
for clinical use. Generally, modii cations of B29 have a less pronounced effect on dimerization
than modii cation of ProB28. The double mutant is especially interesting because it has the same
isoelectric point (p
I
) as human insulin as their amino acid compositions are identical, and thereby
the same solubility. The double mutant called insulin lispro is marketed under the brand name
Humalog.
LysB29
LysB28
*
*
ProB29
ProB28
*
*
(A)
(B)
FIGURE 2.15
(A) Human insulin (pdb-code 1ZNJ, green) and insulin lispro (pdb-code 1LPH, yellow),
showing the change in conformation of the C-terminus (marked by an asterisk). (B) The ProB28-LysB29 in
human insulin and the LysB28-ProB29 in insulin lispro are shown as stick models. Heteroatoms are colored
red and blue for oxygen and nitrogen, respectively. The side-chain nitrogen atom in LysB29 could not be
observed in the x-ray structure.