Biomedical Engineering Reference
In-Depth Information
Sialic acid is also a weak inhibitor of neuraminidase (IC
50
1 mM) and binds to the enzyme in
a half-boat conformation. The weak, nonselective inhibitor 2-deoxy-2,3-didehydro-d-
N
-acetyl-
neuraminic acid (Neu5Ac2en, DANA, Figure 2.5D) was developed as a sialic acid analog resem-
bling the transition state of the enzymatic process (IC
50
= 1-10
∼
M). Based on the structure of the
neuraminidase-sialic acid complex, the complex with DANA and computational studies using the
program GRID, which determines favorable binding sites for probes resembling various functional
groups, it could be shown that replacement of the hydroxy group at the 4-position on DANA by an
amino or a guanidinyl group would enhance binding. It was predicted that a substantial increase
in binding could be obtained by introducing an amino group because a salt bridge was formed to
a negatively charged side chain in the enzyme. Further replacement of the amino group with the
more basic guanidinyl group was as anticipated yielding an even tighter binding inhibitor due to
the formation of salt bridges to two negatively charged side chains in the enzyme. Both the pre-
dicted increase in afi nity and the binding mode were subsequently coni rmed experimentally. The
4-guanidinyl analog (Figure 2.5E) was named zanamivir and was the i rst neuraminidase inhibitor
approved for treatment of inl uenza in humans. It is marketed under the trade name Relenza.
The low oral bioavailability and rapid excretion of zanamivir clearly showed that further improve-
ments were needed in order to obtain a successful anti-inl uenza drug. Based on the 3D structures
of several neuraminidase-inhibitor complexes and computer-based studies, the characteristics of the
active site and thereby the properties of the optimal inhibitor were deduced. Replacing the pyra-
nose ring with a benzene ring reduced the afi nity, showing that the half-boat conformation of the
six-membered ring was important for obtaining a proper orientation of the substituents. Inhibitors
with the pyranose ring replaced by a carbocyclic ring system showed promising afi nities, and by
introducing more lipophilic substituents compounds with signii cantly better oral bioavailability
were obtained.
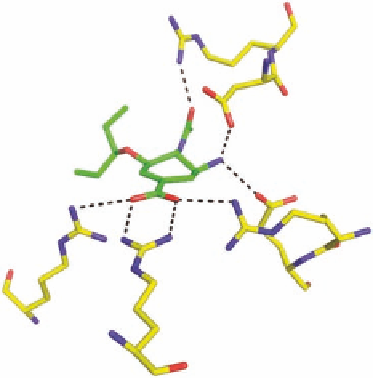
In the i nal active drug candidate GS4071 (IC
50
μ
1 nM) the carbocyclic ring adopts the half-boat
conformation and the polar substituents are all involved in hydrogen bonding to polar residues in the
neuraminidase active site (Figure 2.6). The ethyl ester was named oseltamivir and its phosphate salt
(a prodrug, see Chapter 9) is marketed under the trade name Tamil u.
∼
H
N
O
NH
2
Arg152
O
O
O
Asp151
(A)
Glu119
O
Arg292
H
N
+
Arg118
O
NH
3
Arg351
O
O
-
(B)
FIGURE 2.6
Left: Chemical structures of oseltamivir (A) (oseltamivir phosphate = Tamil u) and the active
component of Tamil u (GS4071) (B) formed by enzymatic cleavage. Right: Hydrogen-bonding network
between (B) and neuraminidase (pdb-code 2QWK).