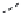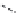Biomedical Engineering Reference
In-Depth Information
25.5.2 I
NHIBITORS
OF
THE
50 S R
IBOSOMAL
S
UBUNIT
25.5.2.1 Chloramphenicol
The i rst broad-spectrum antibiotic was chloramphenicol, which was isolated in 1947 from
Streptomyces venezuelae
. Its chemical structure was soon established and in 1949 a synthesis was
described. The commercial production always has been by the synthetic route. Of the four possible
diastereoisomers, only the
R
,
R
isomer is active and is separated during the synthesis.
Many derivatives of chloramphenicol were prepared but only the sulfomethyl analogue, thiam-
phenicol, has come into clinical use. It is generally less active than chloramphenicol. The glycinate
ester is used as a prodrug for injections. Chloramphenicol has a moderate activity against Gram-
positive and Gram-negative bacteria. It is not recommended for treatment of these infections, because
of the occurrence of serious toxic reactions in the blood (aplastic anemia, thrombocytopenia). It is
still used in the treatment of typhus and meningitis caused by
Haemophilus inl uenzae
.
25.5.2.2 Macrolides
The macrolide antibiotics have in common (a) a large lactone ring (hence the name macrolide); (b) a
glycosidically linked aminosugar (sometimes two), and (c) usually a desoxysugar. The lactone ring
may contain 12 (macrolides not used in medicine), 14 (erythromycin, oleandomycin), and 16 atoms
(leucomycin, spiramycin, tylosin).
The i rst clinical useful macrolide was erythromycin, isolated from a culture of
Streptomyces
erythreus
(1952). The structure was determined by chemical methods (1954-1957) and the
stereochemistry and conformation by x-ray diffraction and NMR. Erythromycin is inactivated
by acid.
Clarithromycin, where the C
6
-hydroxy group is replaced by a methoxy group and roxithromycin,
where the ketone group is under the form of an oxime-ether is more acid-stable (Figure 25.15).
The 9-position of erythromycin has been changed more dramatically in dirithromycin and
azithromycin. Their spectra are comparable to that of erythromycin itself. Azithromycin has a
longer half-life. Recently, telithromycin was introduced as a semisynthetic derivative of erythromy-
cin. It is active a.o. against
S. pneumonia
, b-hemolytic streptococci,
L. pneumophila
, C
hlamydia
pneumoniae
, and
Mycoplasma pneumoniae
(Figure 25.16).
Oleandomycin was isolated in 1955 by Sobin et al. from
Streptomyces antibioticus
, It is admin-
istered usually as the triacetyl derivative, which gives higher blood levels.
R
2
O
D
-Desosamine
D
-Desosamine
H
3
C
CH
3
H
3
C
O
N(CH
3
)
2
N(CH
3
)
2
R
1
HO
O
CH
2
CH
3
H
3
C
HO
OH
O
H
3
C
OR
RO
H
3
C
H
3
C
CH
3
H
3
C
C
2
H
5
O
O
CH
3
O
O
O
CH
3
O
CH
3
O
CH
3
O
O
O
O
CH
3
OR
CH
3
OH
H
3
C
OCH
3
OCH
3
L
-Oleandrose
R
1
R
2
H
= O
Erythromycin
H
= N
Roxitthromycin
O
CH
2
CH
2
OCH
3
Oleandomycin
R = H
CH
3
Clarithromycin
O
R = COCH
3
Troleandomycin
FIGURE 25.15
Macrolides.