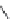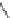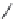Biomedical Engineering Reference
In-Depth Information
O
O
F
COOH
R
1
COOH
R
1
N
R
2
N
N
X
R
2
C
2
H
5
R
1
R
2
R
1
R
2
X
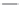
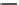
Nalidixic acid
Oloxacin
H
CH
3
N
CH
3
CH
3
N
O
HN
N
F
Enoxacin
Ibaloxacin
CH
3
CH
3
CH
2
H
S
Ruloxacin
N
CH
3
N
(B)
FIGURE 25.9 (continued)
Streptomycin has three basic groups, and it is used usually as the sulfate salt. All aminoglycosides
have a cyclohexane ring moiety viz. in streptomycin diaminotetrahydroxycyclohexane (streptam-
ine) and in others diaminotrihydroxycyclohexane (deoxystreptamine). In streptomycin, the amino
groups of streptamine are replaced by guanidino groups (streptidine). To this ring is attached a
pentose containing an aldehyde group (streptose), to which is linked
N
-methyl-l-glucosamine. All
aminoglycosides present a certain toxicity: vestibular disturbance, with problems of equilibrium,
and cochleotoxicity, which may result in partial and total loss of hearing. Streptomycin, which
causes mainly vestibular toxicity, was replaced at a certain time by dihydrostreptomycin (the alde-
hyde function of the streptose moiety is replaced by an alcohol group). This derivative however had
a greater cochleotoxicity, and it is not used anymore in human medicine.
The second aminoglycoside that was introduced in medicine was neomycin. It was isolated in
1949 by Waksman and Lechevalier from the cultures of
Streptomyces fradiae
. Neomycin is a mix-
ture of components B and C. It was demonstrated that dextromycin, fradiomycin, and framycetin
were identical to neomycin. The last product is mainly neomycin B. Neomycin presents a good
activity against Gram-positive and Gram-negative bacteria but it is very ototoxic. Like other amino-
glycosides, it is very slightly absorbed from the digestive tract, so its oral use does not produce
this systemic toxicity. Neomycin is useful for the treatment of intestinal infections and for external
application (eye drops, eardrops, ointments).
Paromomycin was discovered in 1959 in
Streptomyces rimosus
variant
paromomycinus
. It is also
a mixture of two components like neomycin, and it differs from this antibiotic by the replacement
of one amino group by a hydroxy group. The product was discovered in several laboratories and
catenulin, hydroxymycin, zygomycin, and aminosidin are all paromomycin (Figure 25.10).
Paromomycin sulfate has broad-spectrum antibacterial activity but its use is largely restricted to
the treatment of intestinal amebiasis.
Kanamycin was isolated in 1957 in Japan by Umezawa et al. from
Streptomyces kanamyceticus
.
Kanamycin (used as sulfate, like most aminoglycosides) has four amino groups. Its ototoxicity and
renal toxicity is much lower than that of neomycin and paromomycin. At the time of its introduction,
it was applied mainly for the treatment of infections due to penicillin resistant staphylococci. Now
it is only used externally (Figure 25.11).
Gentamicin and sisomicin are aminoglycosides produced by
Micromonospora
. Gentamicin was
discovered in 1963 in cultures of
Micromonospora purpurea
. The fermentation yields a mixture of