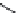Biomedical Engineering Reference
In-Depth Information
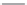
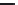
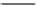
TABLE 25.3
Penicillins
Z
N
S
CH
3
R
1
CO
C
C
CH
3
COOH
N
H
O
Z
R
1
CH
COONa
H
Carbenicillin
CH
COONa
H
Ticarcillin
S
CH
COONa
OCH
3
Temocillin
S
S
CH
3
CO
NH
C
CH
3
Cyclacillin
COOH
N
C
NH
2
H
O
S
CH
3
N
CH
N
C
C
CH
3
COOR
2
N
H
O
R
2
CO
CH
2
O
C(CH
3
)
3
Pivmecillinam
CH
O
COOEt
Bacmecillinam
CH
3
H
Mecillinam
responsible for a rather low activity and had to be replaced by other groups. No microorganism was
found that could enzymatically remove the side chain, but this transformation could be performed
by chemical means. The i rst two cephalosporins introduced in the clinic, cefalothin (1962) and cefa-
loridine (1964), have a thienylacetic acid side chain. Of the thousands of cephalosporins that were
synthesized, only a few reached the clinic (Table 25.4).
They are often classii ed in generations. This classii cation is more or less related to the year
of introduction (I:1962-1971, II:1974-1977, III:1976-1980) and their properties. One should not
consider that cephalosporins of the third generation are superior in all respects to those of the
i rst one.
Generation I cephalosporins are used mainly in infections with Gram-positive bacteria. The
II generation is also active against certain Gram-negative bacteria like
Neisseria
and
Haemophilus
.
The introduction of heterocyclic rings in C
3
is responsible for the metabolic stability of these cepha-
losporins. However, some structures like the tetrazolthiomethyl group (in cefamandole, cefotetan)
are responsible for a disuli ram effect and prolonged blood coagulation. These side effects inl uence
unfavorably their clinical application.