Biomedical Engineering Reference
In-Depth Information
150 nM. According to the ligand-receptor x-ray structure, the binding displays a bidentate charge-
assisted hydrogen bond between the terminal glycerol hydroxy groups and a glutamate side chain.
Removal of the glycerol side chain as in analogue
1.2
and replacement with an hydrophobic alkyl
group increases the afi nity to 1 nM (the minor modii cations of the six-membered ring are not
expected to inl uence the afi nity signii cantly). An x-ray structure of the protein complex (
1.2
)
shows that the glutamate side chain is folded back, opening up a large hydrophobic pocket as
indicated in Figure 1.11.
1.3.5
D
G
vdW
—A
TTRACTIVE
AND
R
EPULSIVE
v
D
W I
NTERACTIONS
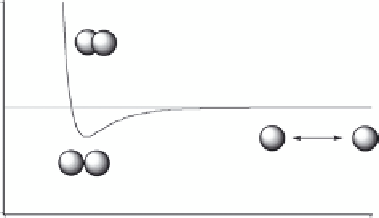
Nonpolar interactions between atoms, that is, vdW interactions, may be attractive as well as repulsive
as shown by the vdW energy curve in Figure 1.12.
At short atom-atom distances, the vdW interaction is repulsive due to overlap of the electron
clouds. The repulsion rises steeply with decreasing atom-atom distance in this region of the energy
curve. When a part of the ligand clashes with atoms in the binding site, this steric repulsive vdW
interaction is responsible for the often dramatic reduction in afi nity that is observed.
At a longer distance, there is a region of attraction between the atoms. This attraction is due to
the so-called dispersion forces. These are basically of electrostatic nature and due to interactions
between temporary dipoles induced in two adjacent atoms. For a single atom-atom contact, the
strength of the interaction is small, ca. 0.2 kJ/mol. However, as the total number of such interactions
may be large, the dispersion interaction may in cases of a close i t between ligand and protein be
signii cant. In this context it should be mentioned that the hydrocarbon tails in the core of a bilayer
membrane are held together by dispersion forces.
As discussed in Section 1.3.5, a methyl group may be expected to increase the afi nity of a
compound by a factor of 3-10 due to the hydrophobic effect. Provided that the methyl group can
be accommodated in the binding cavity. If it cannot be accommodated, vdW repulsion may instead
give a signii cant decrease in afi nity. Thus, the effect on the afi nity of introducing a methyl
group at different positions in a ligand may be a useful strategy to map out the dimensions of a
receptor cavity in lack of experimental information on protein 3D-structure (see the discussion on
pharmacophore modeling in Chapter 3). Provided that the introduced methyl group does not change
the conformational properties of the parent molecule, the changes in afi nity may be interpreted
exclusively in terms of hydrophobic interactions and vdW interactions.
An example of effects that may be observed when introducing methyl groups in a ligand is shown
in Figure 1.13. As discussed in detail in Chapter 3, l avone (
1.3
) binds to the benzodiazepine site of
the GABA
A
receptor. The effects on the afi nity of methyl groups in different positions of the parent
l avone compound (
1.3
) may be interpreted in terms of properties of the binding cavity.
Energy
Repulsion
0
Attraction
r
Distance
FIGURE 1.12
The vdW energy curve.