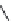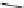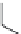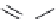Biomedical Engineering Reference
In-Depth Information
O
OH
O
O
N
H
10
5
R1
OH
HN
N
O
H
R2
O
N
N
H
H
2
N
HN
H
A
B
R2 = H; tetrahydrofolic acid
R2 = Formyl; natural cofactor
for GAR FTase
R1
H
2
N
N
N
Pterin ring
Folic acid
(A)
(B)
Sites of modiication
NH
2
O
N
R1
H
N
N
R1
HN
6
R3
H
2
N
N
N
Chirality
H
N
H
2
N
R3 = H; aminopterin
R3 = Me; methotrexate
DDATHF B (lometrexol)
(C)
(D)
O
OH
O
N
O
OH
H
H
2
N
O
N
H
(E)
Alimta
FIGURE 23.3
Chemical structures of various antimetabolites preceding and founding the development of
Alimta.
methylene groups forming
N
5
,
N
10
-methylene tetrahydrofolate from one of the three carbon donors:
formaldehyde, serine, or glycine (Figure 23.4). The key reaction is the TS-catalyzed methylation of
deoxyuridine monophosphate (dUMP) to generate thymidylate (dTMP), which is needed for DNA
synthesis. Methyl tetrahydrofolate (CH
3
THF) is formed from methylene tetrahydrofolate by reduc-
tion of the methylene group and formyl tetrahydrofolate (CHOTHF, folinic acid) results from the
oxidation of the same precursor (Figure 23.4).
Inspired by the active pterine structures (Figure 23.3A), many modii cations were made in this
ring system including modii cations in ring A, such as substitution of NH
2
with methyl or hydrogen
as well as exchange of the fused ring B for a fused phenyl ring. This resulted in compounds having
high biological activity as TS inhibitors with concomitant antiproliferative activity. Some of these
analogs were indeed taken into early clinical testing, but were stopped due to pharmacokinetic or
toxicological problems.
An important new class of potent folate antimetabolites that are active as antitumor agents are
represented by 5,10-dideaza-5,6,7,8-tetrahydrofolic acid (DDATHF, Lometrexol) in which the two
nitrogens in positions 5 and 10 is exchanged for carbon and the B ring is reduced and as such mimic
the structure of THF (Figure 23.3D). The target enzyme for DDATHF was shown to be glycinamide
ribonucleotide formyltransferase (GARFT) (Figure 23.4) catalyzing the i rst folate cofactor-dependent
formyl transfer step in the de novo purine biosynthetic pathway instead of the DHFR enzyme, which
was the target for earlier folate inhibitors described above.
The two diastereomeric forms of DDATHF were separated and their biological activity exam-
ined. Interestingly, they did not show any signii cant difference in activity and further work was
therefore undertaken to remove this chiral center so as to obtain a stereochemically pure compound.