Biomedical Engineering Reference
In-Depth Information
2-13) for a neutral bond and by 10-20 kJ/mol (equivalent to a 50- to 500-fold increase in afi nity)
for a charge-assisted hydrogen bond or a salt bridge.
1.3.3.2 Polar Interactions Involving Aromatic Ring Systems
Other types of polar interactions often observed in ligand-protein complexes are
π
-
π
and cation-
π
interactions. The exact nature of these interactions is quite complex, but qualitatively they can be
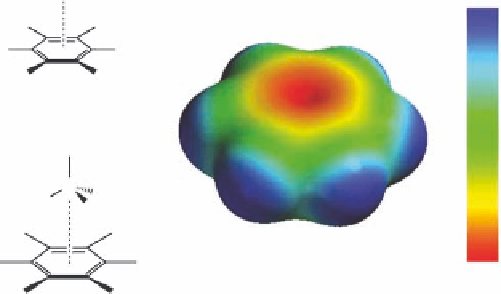
easily understood in terms of electrostatics. Figure 1.8 displays the calculated molecular electrostatic
potential of benzene. It is obtained by calculating the energies of interaction between a benzene ring
and a cation placed in different positions around the aromatic ring. The electrostatic potential in
Figure 1.8 is color-coded on the vdW surface. A red color indicates a strong attraction between the
cation and the aromatic ring and a blue color indicates a strong repulsion. Thus, a cation, for example
an ammonium ion, may favorably interact with the face of the benzene ring, as shown in Figure 1.8.
In
interactions, the edge of one benzene ring interacts with the face of the other.
Other aromatic rings such as phenol and indole display similar electrostatic potentials as ben-
zene. Thus, the aromatic rings of phenylalanine, tyrosine, and tryptophan side chains may favor-
ably interact with positively charged functional groups of the ligand. It has been estimated that a
cation-
π
-
π
interaction may contribute by 8-17 kJ/mol to the overall binding of the ligand, which is
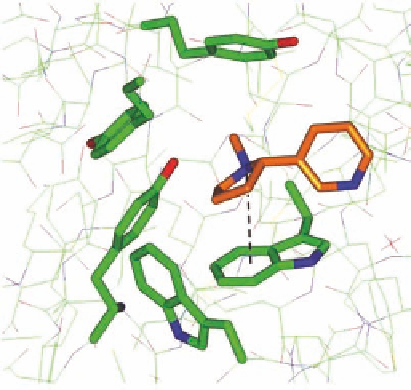
equivalent to a 23- to 760-fold increase in afi nity. Figure 1.9 shows the binding of nicotine to the
π
+
12.706
H
H
H
H
7.226
H
H
1.747
-3.733
-9.212
+
N
CH
3
CH
3
H
3
C
-14.692
H
H
-20.171
H
H
H
H
FIGURE 1.8
The molecular electrostatic potential of benzene.
H
N
+
+
N
Nicotine
Trp 143
FIGURE 1.9
Nicotine in the binding pocket of the AChBP (pdb-code 1UW6).