Biomedical Engineering Reference
In-Depth Information
O
Neutral
H
O
+
Charge assisted
NH
3
O
H
N
H
O
Charge-assisted
“salt bridge”
HN
-
O
N
H
H
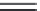
FIGURE 1.6
Examples of different types of hydrogen bonds observed in ligand-protein complexes.
O
O
-
-
O
O
+
NH
3
(
S
)-Glutamate
FIGURE 1.7
The binding of (
S
)-glutamate to the ligand-binding domain of the ionotropic glutamate recep-
tor iGluR2 (pdb-code 1FTJ).
(Figure 1.2) and that hydrogen bonding is an exchange process. Before formation of the ligand-protein
complex (left-hand side in Figure 1.2) the polar functional groups of the ligand, the polar amino acid
residues and C
O and NH backbone groups in the protein are engaged in hydrogen bonding with
surrounding solvent water molecules. In the ligand-protein complex (right-hand side in Figure 1.2)
these hydrogen bonds to the solvent are replaced by hydrogen bonds between the ligand and the
protein. The net effect of this hydrogen bond exchange process is the difference in free energy
between hydrogen bonding to water and to the protein. As a consequence of this exchange process,
a substituent that is hydrogen bonded to water molecules in the aqueous phase, but that is buried in
the binding cavity but not hydrogen bonded to the protein (an unpaired hydrogen bond) is strongly
unfavorable for binding. It has been shown that the energy cost for an unpaired hydrogen bond is
ca. 4 kJ/mol for a neutral substituent and ca. 16 kJ/mol for a charged substituent. This is equivalent
to a loss of afi nity by a factor of 5 and 500, respectively.
The successful formation of a hydrogen bond in the binding cavity has been estimated to con-
tribute to the binding afi nity by 2-6.5 kJ/mol (corresponding to an afi nity increase by a factor of