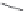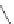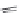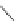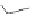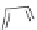Biomedical Engineering Reference
In-Depth Information
Heme
Heme
CH
2
CH
3
O
-
CH
3
N
-
H
3
C
Fe
2+
N
O
N
Digestion
H
2
C
N
-
Heme
Heme
H
3
C
OH
O
Heme (
21.5
)
H
2
C
Hemoglobin
H
3
C
Oxidation
H
3
C
N
-
CH
3
HO
N: Fe
3+
:
N
CH
2
CH
2
O
N
-
CH
3
O
CH
2
CH
3
O
-
CH
3
CH
3
O
-
N
-
H
3
C
Precipitation
O
-
CH
3
Fe
3+
N
O
N
N
-
H
3
C
H
2
C
N
-
N
O
Fe
3+
N:
R
1
H
2
C
N
-
Hemozoin
H
3
C
OH
O
OH
H
3
C
O
Hematin
21.6
FIGURE 21.7
Digestion of hemoglobin, which is a tetramer consisting of four protein strings and four heme
(
21.5
) molecules, leads to liberation of the heme molecules. Oxidation of iron(II) to iron(III) converts heme into
hematin. Ionic interactions between the propanoate side chain and the iron(III) yield the poorly soluble b-hematin
= hemozoin (
21.6
), which precipitates (shown as a dimer, but in the cell hemozoin will precipitate as a polymer).
CH
3
CH
3
CH
3
CH
3
N
CH
3
N
CH
3
H
HN:
N
+
5
4
6
3
7
2
N
-
N
N
Cl
Cl
8
1
The quinoline nucleus
Chloroquine
21.7
FIGURE 21.8
The quinoline nucleus and coni gurations of chloroquine (
21.7
) and hydroxychloroquine and
resonance structures revealing the delocalization of the electrons in the electron-rich pyridine ring. Protonation
of the two basic amino groups ensures accumulation in the food vacuole.
The lipophilicity of the neutral molecule enables it to cross the membranes of the erythrocytes and
the parasites. Having entered the food vacuole with a pH ~ 5.2, the two amino groups will become
protonated preventing chloroquine from leaving the vacuole by passive penetration. Furthermore,
the protonation of quinoline nitrogen enables a cation-p interaction between the quinoline and the
hemozoin (Figure 21.9).