Biomedical Engineering Reference
In-Depth Information
600
500
Zolpidem
Zopiclone
500
400
400
300
300
200
200
100
100
0
0
0,001
0,01
0,1
1
10
100
0,001
0,01
0,1
1
10
100
Zolpidem (μM)
Zopiclone (μM)
500
500
Indiplon
400
400
Zaleplon
300
300
200
200
100
100
0
0
0,001
0,01
0,1
1
10
100
0,00001 0,0001 0,001
0,01
0,1
1
10
Zaleplon (μM)
Indiplon (μM)
α
1
β
3
γ
2
α
2
β
3
γ
2
α
3
β
3
γ
2
α
5
β
3
γ
2
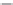
M GABA by a series of BzRAs at therapeutic relevant concentrations. The
therapeutic relevant concentration is marked with a blue box. Dose response curves are from in vitro func-
tional experiments in
Xenopus
oocytes. (Adapted from Petroski, R.E. et al.,
J.
Pharmacol. Exp. Ther
., 317,
369, 2006.)
FIGURE 20.6
Modulation of 3
μ
use of these BzRAs. The reason for the discrepancy between animal and human data most likely
relates to differences in levels and duration of exposure. Most preclinical studies are conducted with
the aim of demonstrating a certain pharmacological effect. Therefore most tolerance development
studies and receptor down regulation studies are carried out with constant and high exposure, which
under normal circumstances may be highly irrelevant from a clinical perspective, but certainly
addresses aspects of the mechanisms underlying tolerance development. In contrast, therapeutic
exposure with hypnotics usually last for only a few hours per 24 h and the peak concentration is
selected as a compromise between optimal effectiveness and side effects. The duration of exposure
relative to nonexposure is therefore very small and this may allow resensitization of desensitized
receptors and prevent signii cant down regulation.
This should also be borne in mind with the development of sustained release formulations, in that
tolerance development may be more likely to occur with this type of therapy, where receptors are
exposed to the drug for longer. Since these are relatively new to the market, no clinical data has yet
been published on these new formulations.
Preclinical studies have, as indicated above, consistently demonstrated that BzRAs are abus-
able. Since all hypnotic BzRAs possess a very strong afi nity for a1 containing receptors, it has
been assumed that this subunit drives both abuse liability and hypnotic effects. If this indeed is the
case, BzRA-based compounds will always be associated with this problem. However, as illustrated
above, at clinically meaningful concentrations, BzRAs all show signii cant activities at a2 and a3