Biomedical Engineering Reference
In-Depth Information
are known. Agonists are primarily applied as analgesic, anesthetic, antitussive, and in the treat-
ment of diarrhea. Morphine and codeine are mostly used as analgesics. Fentanyl is a very potent
analgesic and used in anesthesia. Meperidine is used for acute pain. Methadone is applied to
control the withdrawal of heroin from addicts. Antagonists are used for the reversal of some of
the effects induced by agonists. Thus, naloxone has been used to reverse coma and respiratory
depression of opioid overdose (methadone and heroin). It is also indicated as an adjunct agent
to increase blood pressure under septic shock. Naltrexone has been approved as an adjunctive
therapy in the treatment of alcohol dependence and the treatment of narcotic addiction to opioids.
However, there are also potential indications including obesity, obsessive compulsive disorder,
and schizophrenia.
19.2 CANNABINOID RECEPTORS
Different parts of the plant
Cannabis sativa
has for millennia been used for recreational and medici-
nal purposes, as it can be seen from old Chinese, Assyrian, and Roman literature. However, it was
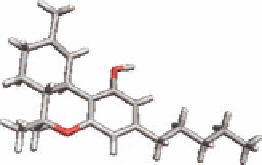
i rst in 1964 that the active principle causing the psychoactive effects was isolated and found to be
D
9
-tetrahydrocannabinol (THC) (Figure 19.8). Originally, it was thought that THC due to its lipophi-
licity somehow acted through l uidizing the cellular membranes, but in the early 1990s it was dis-
covered that THC activates two receptors, cannabinoid receptor-1 (CB
1
-receptor) and cannabinoid
receptor-2 (CB
2
-receptor). Cannabinoid effect in rodents is characterized by the so-called tetrad test.
In this test, measurement of spontaneous activity, thermal pain sensation, catalepsy, and rectal tem-
perature are made, and compounds with cannabinoid activity should produce hypomotility, analgesia,
catalepsy, and hypothermia. Shortly after the discovery of the receptors, two endogenous compounds
were identii ed that could activate these receptors, i.e., anandamide (
N
-arachidonoylethanolamine
or arachidonoylethanolamide) and 2-arachidonoylglycerol (2-AG) (Figure 19.9), and they are called
endocannabinoids. Both the endocannabinoids are lipids and thus not very water soluble. They may
associate with albumin or lipoproteins in the extracellular space and endocannabinoids function in
an autocrine and paracrine fashion where they are formed “on demand” and then degraded, i.e., they
are not stored in vesicles like neurotransmitters or peptide hormones. Tissue levels of anandamide
and 2-AG are usually in the pmol/g and nmol/g tissue, respectively, but it is not clear whether these
levels represent the ligand concentration available to the receptors. However, it is generally consid-
ered that there is an endogenous tone of endocannabinoid level in most tissues. Endocannabinoids
are also found in very small concentrations in plasma where they are thought to represent spill over
from the tissues.
19.2.1 E
NDOCANNABINOID
S
YSTEM
The endocannabinoid system comprises the two cannabinoid receptors, the two endocannabinoids
and the enzymes that synthesize and degrade the endocannabinoids. Biosynthesis of anandamide is
complex, but it is formed from an unusual phospholipid having three fatty acids,
N
-arachidonoyl-
phosphatidylethanolamine that again is formed from phosphatidylethanolamine by the acyla-
tion of the amino group catalyzed by a poorly known calcium-stimulated
N
-acyltransferase.
O
Anandamide
R
OH
OH
R =
H
2-Arachidonylglycerol
O
OH
R =
OH
Tetrahydrocannabinol (THC)
O
FIGURE 19.8
Plant cannabinoid (THC) and two endocannabinoids.