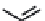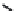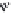Biomedical Engineering Reference
In-Depth Information
The introduction of a chloro substituent into the template structure
18.36
further increased
5-HT reuptake and decreased NE reuptake inhibition (
18.36
and
18.38
), in accordance with
observations by Carlsson that halogen substituents in both zimelidine (
18.47
, Figure 18.6) (see the
following text) derivatives and in of imipramine (clomipramine,
18.25
, Figure 18.4) increased
5-HT reuptake. Indeed, the dichloro derivative
18.39
proved to be a selective 5-HT reuptake
inhibitor. So the goal of obtaining an SSRI from an SNI was achieved very fast (in 1971), when
less than 50 compounds had been synthesized.
The SAR were further explored, and it was established that high activity was generally found in
5,4¢-disubstituted compounds where both substituents were halogen or other electron-withdrawing
groups. Cyano-substituted compounds were obtained by the reaction of the bromo precursors (e.g.,
18.40
) with CuCN. One of the cyano-substituted compounds was (
18.43
), later known as citalopram
(INN name). The compound was synthesized for the i rst time in August 1972. The cyano group
could be metabolically labile, but it was subsequently shown not to be the case neither in animals
nor in humans. Citalopram displayed the best overall preclinical proi le within this series and was
consequently selected for development. The 5-cyano substituent in citalopram also proved to be
chemically stable in a surprising manner; for example, it does not react with Grignard reagents,
which has led to a new and patentable process for its production.
Citalopram was launched in Denmark in 1989, and it has since been registered worldwide.
Citalopram is a racemate, having an asymmetric carbon at the 1-position. When it was synthesized
in 1972, classical resolution via diastereomeric salts was the only realistic alternative for separation
of the enantiomers. However, it is generally difi cult to make salts of citalopram, and eventually
direct resolution was given up. Finally, an intermediate was resolved in this way, and the resolved
intermediate could then be transformed into the pure (
S
)- and (
R
)-enantiomers of citalopram.
Subsequent testing showed that all the 5-HT reuptake inhibition resided in the (
S
)-enantiomer. The
high stereospecii city was later rationalized in the SSRI pharmacophore model discussed in the
following text. The (
S
)-enantiomer (INN name escitalopram) has subsequently emerged as a new
drug dei ning a new group of antidepressants, namely the ASRIs. The discovery and its implications
are discussed in the following text.
N
NC
F
3
C
F
3
C
O
CH
3
CH
3
O
N
N
NH
2
O
O
O
O
NHCH
3
OCH
3
F
F
Paroxetine (
18.45
)
30.1.1973
Fluvoxamine (
18.46
)
20.3.1975
Citalopram (
18.43
)
14.1.1976
Fluoxetine (
18.44
)
10.1.1974
Cl
N
CH
3
CH
3
N
Cl
NHCH
3
NH
HN
Br
Indalpine (
18.48
)
12.12.1975
Zimelidine (
18.47
)
28.04.1971
Sertraline (
18.49
)
1.11.1979
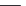
FIGURE 18.6
Selective serotonin reuptake inhibitors (SSRIs).