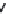Biomedical Engineering Reference
In-Depth Information
TABLE 18.1
Receptor Profi le and EPS Potential of Antipsychotic Drugs
In Vivo
ED
50
(
m
mol/kg)
Receptor Binding
K
i
(nM)
Catalepsy
Max., sc
a
D
a
D
a
D
b
D
b
5-HT
2
a
5-HT
2
a
α
a
Compounds
Classical antipsychotic drug
Haloperidol (
18.9
)
15
0.82
1.1
2.8
28
1500
7.3
0.34
Atypical antipsychotic drugs
Risperidone (
18.13
)
21
0.44
14
7.1
0.39
6.4
0.69
17
Olanzapine (
18.11
)
10
2.1
71
32
1.9
2.8
7.3
37
Quetiapine (
18.12
)
390
69
1100
2400
82
1500
4.5
>80
Ziprasidone (
18.14
)
9.5
2.8
n.t.
73
0.25
0.55
1.9
>48
Sertindole (
18.16
)
12
0.45
2.0
17
0.20
0.51
1.4
>91
Clozapine (
18.10
)
53
36
310
30
4.0
5.0
3.7
120
Sources:
Data from
a
Arnt, J. and Skarsfeldt, T.
Neuropsychopharmacology
, 18, 63, 1998;
b
Lundbeck Screening
Database, H. Lundbeck A/S, Valby, Denmark.
Note:
n.t; not tested.
DA system, inhibiting transmission in synapses with high tonus, and increasing function in those
with low activity. This proi le might explain why aripiprazole does not induce EPS.
18.2.1.2.1 Discovery of Sertindole
In the 1970s, Lundbeck had successfully marketed a number of classical antipsychotic drugs, and the
medicinal chemistry program at Lundbeck was still aimed at i nding new antipsychotic drugs based on
the phenothiazine or thioxanthene template. However, in 1975, the i rst compounds were synthesized
in a project directed toward the identii cation of nonsteroidal anti-inl ammatory drugs (NSAIDs),
and fortunately the compounds were also examined in
in vivo
models predictive of antipsychotic and
antidepressant action. It was found that the two
trans
-1-piperazino-3-phenylindanes
18.17
and
18.18
(Figure 18.3) were relatively potent in the methyl phenidate-induced hyperactivity model (predictive
of antipsychotic effect/mechanistic model for D
2
antagonism), but at the same time about a factor of 10
weaker in the catalepsy model (predictive of EPS). Thus, these compounds were seen as prototypes of
a new class of antipsychotic drugs with an improved side effect proi le with respect to EPS.
In 1980, the
trans
-racemate tel udazine (
18.19
, Figure 18.3) was selected from this series as
a development agent with potential antipsychotic effect. Tel udazine displayed a similar ratio in
the methyl phenidate hyperactivity versus the catalepsy model as the two lead compounds, but
F
F
F
3
C
F
F
F
F
N
N
N
N
N
N
N
N
H
3
C
O
HO
HO
N
HN
18.17
18.18
Teludazine (
18.19
)
Irindalone (
18.20
)
FIGURE 18.3
Selected
trans
-1-piperazino-3-phenylindanes.