Biomedical Engineering Reference
In-Depth Information
present in the synapse, and thus the stimulation of the receptors introduced by the allosteric modula-
tor occurs in a physiological tone. Second, since the allosteric ligands target regions less conserved
than the orthosteric site in different nAChR subtypes, they are more likely to be subtype-selective
than orthosteric ligands. Finally, in contrast to agonists, which for the most parts mimic the activa-
tion kinetics of the endogenous agonist, allosteric potentiators can enhance nAChR signaling in
many different ways, for example, by increasing the ion conductance of the receptor, by increasing
the frequency of ACh-induced ion channel openings, or by reducing the desensitization rate.
The urea analogs PNU-120596 (
16.78
) and NS-1738 (
16.79
) are selective allosteric potentiators
of the a
7
nAChR. Both compounds enhance the potency of as well as the maximal response elicited
by ACh through the receptor, having no effect on receptor signaling in the absence of ACh. In addi-
tion to these effects,
16.78
also suppress the desensitization of a
7
(Figure 16.15) and can restore the
activity in an already desensitized receptor. In contrast to the subtype-selective activities of these
potentiators, LY-2087101 (
16.80
) potentiates the signaling of a
2
b
4
, a
4
b
4
, a
4
b
2
, and a
7
nAChRs
but not that of the muscle-type, a
3
b
2
, and a
3
b
4
subtypes. Interestingly, the AChEIs physostigmine
(
16.11
) and galanthamine (
16.15
) have also been shown to potentiate the ACh-evoked responses
through several nAChR subtypes.
16.5.4 L
IGAND
B
INDING
TO
THE
nAChRs
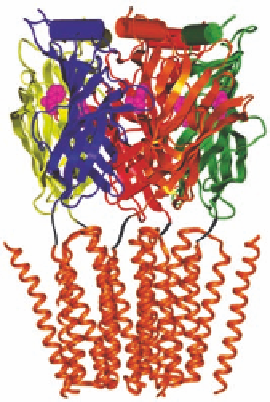
The orthosteric sites of the nAChR are situated in the extracellular amino-terminal domain of
the pentameric receptor complex, more specii cally at the interfaces between a- and b-subunits
in the heteromeric nAChR and between two a-subunits in the homomeric nAChR (Figure 16.16).
Thus, the heteromeric and homomeric nAChRs contain two and i ve orthosteric sites, respectively
(Figure 16.12). Similarly to the mAChR ligands, agonists, and almost all competitive antagonists
Orthosteric ligands
V108
Allosteric ligands
I106
M116
Galanthamine
I118
Y195
C191
C190
PNU-282987
W147
Ca
2+
Y93
Y55
Zn
2+
Y188
Aβ
42
Epibatidine
M116
C190
Q57
Steroids
I118
C191
Mecamylamine
Y195
Y188
W147
S167
Y55
Y93
The nAChR
D197
MLA
FIGURE 16.16
Ligand binding to the nAChR. Right: The binding modes of the agonist epibatidine and the
competitive antagonist MLA to the orthosteric site in the amino-terminal domain of the receptor. (Part of
the i gure is reprinted from Hansen, S.B. et al.,
EMBO J
., 24, 3635, 2005. With permission.) Left: Allosteric
modulators targeting the amino-terminal and ion channel domains of the nAChR complex. (Part of the i gure
is reprinted from Jensen, A.A. et al.,
J. Med. Chem
., 48, 4705, 2005. With permission.)