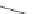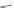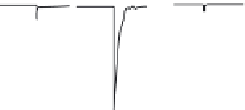Biomedical Engineering Reference
In-Depth Information
for native a
4
b
2
* nAChRs versus a
3
b
4
* and a
7
receptors in binding assays. The epibatidine analog
16.75
is an antagonist with a
K
i
value of 1 pM to native a
4
b* nAChRs, making it the nAChR ligand
with the highest binding afi nity published to date.
Within the last decade, several small peptides of 12-20 amino acid residues, the so-called
a-conotoxins, have been isolated from a family of predatory cone snails, the
Conus
snails. The
peptides have turned out to be very interesting pharmacological tools, as they are highly subtype-
specii c in their antagonism of nAChRs. The 3D structures of the a-conotoxins are established
by intramolecular disuli de bonds formed by the four highly conserved cysteine residues in the
peptides, which are organized in different arrangements: a
3/5
, a
4/3
, a
4/7
, or a
4/6
, the nomenclature
referring to the number of residues between the conserved cysteines (Figure 16.14). The subtype-
selective activities of the respective a-conotoxins arise from the differences in the nonconserved
residues. So far, a-conotoxins selective for nAChR subtypes a
7
(for example ImI), a
3
b
2
(for
example MII and PnIA), and a
3
b
4
(AuIB) have been identii ed (Figure 16.14). Considering that
the entire mollusk family is estimated to contain ~50,000 neuropharmacologically active toxins,
additional subtype-selective nAChR antagonists are likely to be identii ed in the future.
16.5.3 A
LLOSTERIC
M
ODULATORS
OF
THE
nAChRs
As it is the case with other ligand-gated ion channels, such as GABA
A
and NMDA receptors (see
Chapter 15), the nAChRs are highly susceptible to allosteric modulation (see Chapter 12). Several
endogenous ligands, such as steroids (for example
16.76
), 5-hydroxyindole (
16.77
), and Ca
2+
and
Zn
2+
ions modulate signaling through the receptors (Figure 16.15). It is highly interesting to note
that the Ab
42
peptide has been found to be a potent noncompetitive inhibitor of a7 nAChR signaling
but the signii cance of this inhibition for AD remains to be elucidated.
Allosteric potentiators hold several advantages to regular agonists when it comes to the augmen-
tation of nAChR signaling. First, analogous to the AChEIs they only exert their effect when ACh is
OH
O
N
H
N
H
H
N
N
O
O
O
H
H
HO
5-Hydroxyindole (5-HI,
16.77
)
Cl
17 -Estradiol (
16.76
)
PNU-120596 (
16.78
)
OH
H
N
H
N
O
CF
3
N
NH
F
S
O
H
Cl
Cl
S
NS-1738 (
16.79
)
LY-2087101 (
16.80
)
Mecamylamine (
16.81
)
ACh 100 μM (3s)
ACh 100 μM (3s)
10 μM PNU-120596
1 mM 5-HI
2 μA
10 s
FIGURE 16.15
Chemical structures of allosteric modulators of nAChRs and the potentiation of the
ACh-induced a
7
nAChR signaling exerted by 5-hydroxyindole (5-HI) and PNU-120596. (Part of the i gure is
reprinted from Bertrand, D. and Gopalakrishnan, M.,
Biochem. Pharmacol
., 74, 1155, 2007. With permission.)