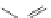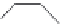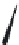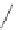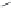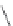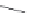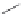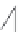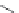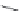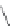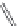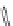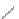Biomedical Engineering Reference
In-Depth Information
16.5.1 nAChR A
GONISTS
As in the case of the AChE and mAChR i elds, medicinal chemistry development of nAChR ligands
has been greatly inl uenced by the plethora of ligands isolated from natural sources. From a therapeu-
tic perspective, there has been most interest in ligands augmenting nAChR signaling (i.e., agonists or
allosteric potentiators). The classical nAChR agonists
16.52 -16.57
have all been isolated from natural
sources, and while they are selective for the nAChRs over the mAChRs, they are rather nonselective
within the nAChR family (Figure 16.13). Because of their potent agonism and high BBB penetration,
(
S
)-nicotine (
16.52
) and (
)-epibatidine (
16.53
) have been the lead compounds for the vast majority
of nAChR agonists developed over the years. Modii cations of the linker between the pyridine and
pyrrolidine rings and introduction of new ring systems in
16.52
have resulted in several potent nAChR
agonists, such as compounds
16.58 -16.60
. Ring-opening of the pyrrolidine ring in (
S
)-nicotine has
given rise to ispronicline (
16.61
) and several other potent analogs. Interestingly, these agonists dis-
play signii cant functional selectivity for the a
4
b
2
over all other nAChR subtypes and have entered
clinical development as cognitive enhancers and analgesics. The 5-ethynyl nicotine analog, altinicline
(
16.62
), is a partial agonist characterized by a modest functional preference for the a
4
b
2
nAChR over
b
4
- containing subtypes.
16.62
is a highly efi cacious stimulant of dopamine release in nucleus accum-
bens and striatum and has been in clinical trial for Parkinson's disease.
(
±
)-Epibatidine (
16.53
) was originally isolated from the skin of the Ecuadorian frog
Epipedobates
tricolor
and is by far the most potent of the classical nAChR agonists. The therapeutic interest in
the compound was founded in studies showing that it is a nonaddictive analgesic, blocking pain
200 times more effectively than morphine. Because of the severe hypertension and neuromuscular
paralysis observed upon epibatidine administration, however, several epibatidine analogs have been
developed in the hope to isolate the analgesic effects from the side effects. Methylation of the basic
amino group and substitution of the 2-chloropyridine ring in
16.53
with a 2-(pyridazin-4-yl) ring
±
N
O
Cl
N
HN
N
N
N
N
N
O
N
(±)-Epibatidine (
16.53
)
(-)-Cytisine (
16.54
)
Anabaseine (
16.55
)
(+)-Anatoxin-a (
16.56
)
(
S
)-Nicotine (
16.52
)
O
OH
R
S
O
O
NH
H
H
N
O
N
Cl
N
N
Lobeline (
16.57
)
ABT-089 (
16.58
)
ABT-418 (
16.59
)
Tebanicline (
16.60
)
Cl
N
O
N
N
HN
O
N
N
N
O
N
Altinicline (
16.62
)
N
Ispronicline (
16.61
)
(
16.63
)
(±)-UB-165 (
16.64
)
N
Cl
O
N
N
O
N
HN
N
NH
GTS-21 (
16.66
)
N
N
N
N
TC-1698 (
16.69
)
O
Varenicline (
16.65
)
AR-R-17779 (
16.67
)
PNU-282987 (
16.68
)
FIGURE 16.13
Chemical structures of nAChR agonists.